Determination of lipid content and stability in lipid nanoparticles using ultra high-performance liquid chromatography in combination with a Corona Charged Aerosol Detector
Color online: See article online to view Figs. 2–4 in color.
Abstract
For many years, lipid nanoparticles (LNPs) have been used as delivery vehicles for various payloads (especially various oligonucleotides and mRNA), finding numerous applications in drug and vaccine development. LNP stability and bilayer fluidity are determined by the identities and the amounts of the various lipids employed in the formulation and LNP efficacy is determined in large part by the lipid composition which usually contains a cationic lipid, a PEG-lipid conjugate, cholesterol, and a zwitterionic helper phospholipid. Analytical methods developed for LNP characterization must be able to determine not only the identity and content of each individual lipid component (i.e., the parent lipids), but also the associated impurities and degradants. In this work, we describe an efficient and sensitive reversed-phase chromatographic method with charged aerosol detection (CAD) suitable for this purpose. Sample preparation diluent and mobile phase pH conditions are critical and have been optimized for the lipids of interest. This method was validated for its linearity, accuracy, precision, and specificity for lipid analysis to support process and formulation development for new drugs and vaccines.
Abbreviations
-
- CAD
-
- charged aerosol detection
-
- DSPC
-
- 1,2-Distearoyl-sn-glycero-3-phosphocholine
-
- LNP
-
- lipid nanoparticles
1 Introduction
Lipid nanoparticles (LNPs) have been used for a variety of applications throughout the years such as therapeutics [1], delivery system [2], vaccines [3], and adjuvants [3]. Most recently, mRNA and LNP technology is being evaluated and approved by the Food and Drug Association (FDA) as a delivery vehicle for two of the leading vaccine candidates for SARS-CoV-2 global pandemic [4]. In the past, this technology was utilized for RSV vaccine candidates such as V171 (mRNA-1777) [5] and preclinical vaccine development for VZV (mRNA-1278) [6]. For any of these applications, the LNP formulation typically consists of multiple lipids.
In this paper, we consider LNPs for mRNA delivery formulated with four types of lipids: a cationic lipid with an amine functional group that interacts with mRNA via ionic interactions (Cationic Lipid 1 or Cationic Lipid 2), a PEG (Polyethylene glycol)-lipid conjugate (PEG-DMG (1,2-Dimyristoyl-rac-glycero-3-methylpolyoxyethylene) or PEG Lipid 2), cholesterol, and a zwitterionic helper phospholipid (1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC)). Quantitation of the individual lipids is required to support process and formulation development and are key quality attributes for manufacture. Chemical stability of each lipid is also important for clinical and safety studies and quality assurance. Lipid chemical degradation could lead to LNP aggregation and degradation during storage [7, 8]. Therefore, a robust, stability-indicating analytical tool was required to support clinical development and the following commercialization.
Previously, gas chromatography coupled with flame ionization detection (GC/FID) [9], LC/MS [10], high-performance liquid chromatography (HPLC), and ultra-high-performance liquid chromatography (UHPLC) coupled with an evaporative light scattering detector (ELSD) [11, 12] have been utilized for the analysis of lipids. However, GC–FID is rather laborious involving derivatization and is not able to analyze high molecular weight components. LC–MS is expensive and difficult for high sample throughput, and is better suited for investigational purposes instead of routine testing. Finally, ELSD does not provide sufficient sensitivity for impurity detection.
Charged aerosol detection has become increasingly popular in the pharmaceutical industry due to its ability to detect analytes lacking chromophores that have low vapor pressure [13]. Previously at Merck & Co., Inc., Kenilworth, NJ, USA, a UHPLC coupled with charged aerosol detector (CAD) method was developed and used for lipid analysis for small interfering RNA (siRNA) LNP studies [14]. The CAD's high sensitivity makes it an ideal choice over the ELSD. Charged aerosol detection has been found to be up to six times more sensitive than the ELSD making integration and identification of low-level impurities better achievable [15, 16]. By utilizing reversed phase liquid chromatography coupled with a CAD, this method could even be modified for mass spectrometry for investigational impurity identification [17]. Finally, the CAD's ease of use and low maintenance is beneficial for transfer to QC labs.
For the aforementioned reasons, UHPLC combined with a CAD was chosen and evaluated to support various mRNA-LNP vaccine programs. In this article, we describe the development of a robust and efficient UHPLC-CAD method using commercially available materials and instrumentation is described for lipid analysis of mRNA loaded lipid nanoparticles used in vaccine candidates. Though three of the lipids mentioned in this article are proprietary and cannot be disclosed, this methodology is valuable to highlight in the growing field of mRNA-LNP technology. In addition to chromatography conditions, sample preparation diluent is also discussed due to its importance for accurate lipid quantitation. This method was validated using standard analytical parameters such as linearity, specificity, range, accuracy, and precision. Finally, an example lipid stability study is reported using the UHPLC-CAD method.
2 Materials and methods
2.1 Chemicals and reagents
Methanol (LCMS grade), 2-propanol (LCMS grade), and Triethylamine (HPLC grade) were purchased from Fisher (Fair Lawn, NJ). Acetic Acid (99.7%+) was purchased from Acros (West Chester, PA). Formic Acid: Triethylamine Complex (5:2), pure ethanol (200 proof), 1-tetradecanoic acid–myristic acid (99% grade), formamide (99% grade), 30% hydrogen peroxide, sodium hydroxide (10.0N) were purchased from Sigma-Aldrich (St. Louis, MO). Cholesterol (99%) was purchased from Minakem (Dunkerque, FR). 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC) (>99%), 1-stearoyl-2-hydroxy-sn-glycero-3-phosphocholine (1-LysoPC), and 2-stearoyl-sn-glycero-3-phosphocholine (2-LysoPC) were purchased from Avanti Polar Lipids (Alabaster, AL). 1,2-Dimyristoyl-rac-glycero-3-methylpolyoxyethylene (PEG-DMG), was purchased from NOF (White Plains, NY). Water was dispensed using a MilliQ filter system from Millipore (Burlington, MA). Octadecanoic acid (referred to as stearic acid) was purchased from European Pharmacopeia (Strausburg, FR). Cationic Lipid 1, Cationic Lipid 2, and PEG Lipid 2 are proprietary products that were manufactured at Merck & Co., Inc., Kenilworth, NJ, USA which operates as MSD outside of the USA and Canada. The LNP vaccine samples were prepared as previously described [18]. In the LNP vaccine manufacture process, a lipid mixture was prepared by dissolving cationic lipid, cholesterol, phospholipid, and PEG-conjugate lipid (molar ratio of 50–58:30–39:10:1–2) in ethanol. Then lipid nanoparticles were produced by simultaneous T-mixing of the lipid mixture with an aqueous solution mRNA, followed by stepwise dia-filtration [19]. Both starting solutions are submitted for analysis along with samples post diafiltration. Each submitted sample has a targeted mRNA dose concentration and is used for sample preparation.
2.2 Assay stock standard and LNP sample preparation
Each lipid was individually dissolved in pure ethanol for stock standard preparation and then diluted with assay diluent (described below). Each LNP sample was treated with a diluent consisting of ethanol:formamide (85:15 %V/V) mixture. A minimum of four-fold dilution relative to the concentration of mRNA is required to fully recover all the lipids based on developmental data (see Section 3.1 for more sample preparation information).
When analyzing samples containing Cationic Lipid 1, 0.5% Formic Acid:Triethylamine Complex (5:2) was added to the assay diluent. When analyzing samples containing Cationic Lipid 2, 19.2mM glacial acetic acid and 16.2mM triethylamine were added to the assay diluent. Samples were diluted to final concentrations that were within the prepared calibration curves.
2.3 Mobile phase composition
When measuring LNP samples containing ionizable Cationic Lipid 1, the following mobile phase compositions were used: mobile phase A consisted of water:methanol (1:1 %V/V) with 0.5% Formic Acid:Triethylamine Complex (5:2) – a premixed, purchasable reagent from Sigma Aldrich – at pH of 3.5, and mobile phase B was composed of methanol with 0.5% Formic Acid:Triethylamine Complex (5:2). When measuring LNP samples containing Cationic Lipid 2, the following mobile phases were used: mobile phase A was composed of water:methanol (1:1 %V/V) with 19.2 mM glacial acetic acid and 16.2 mM triethylamine, and mobile phase B was composed of methanol with 19.2 mM glacial acetic acid and 16.2 mM triethylamine. The pH of both mobile phases was measured after mixing by adding 1mL of mobile phase and 9 mL water, and adjusted with their respective acid or triethylamine when necessary.
2.4 UHPLC-CAD conditions
Samples were analyzed on a Waters Acquity Classic binary UPLC system (Waters Corp, Willmington, DE). Strong needle wash was composed of 2-propanol. Weak needle wash composed of water:methanol (80:20 %V/V). Seal wash was composed of water:2-propanol (1:1 %V/V).
Samples were injected using a Waters Acquity Classic autosampler using a fixed loop injection at 5 μL with a 50 μL sample loop. Lipids were separated using a Waters BEH C18 1.7 μm particle size, 2.1 mm ID x 150 mm column heated to 50°C (Waters Corp, Willmington, DE). Using a flow rate of 0.3mL/min, the mobile phase composition began at 0% B with one-minute hold time, then %B increased to 79% as the first step gradient change. At 7.5 min, %B increased to 98% as the second step gradient change; at 17.5 min, the %B decreased to the initial condition, 0%, and equilibrated for 3.5 min before the next sample injection. Analytes were detected using a Corona VEO RS CAD from Thermo Scientific (Waltham, MA). Filtered nitrogen was used at a preset manufacture pressure of 60.7 psi; data collection rate of 5 Hz; power function of 1; nebulizer temperature set to 35°C. Data were processed using Waters Empower 3 software.
2.5 Theoretical Log D calculations
Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994–2020 ACD/Labs) was used to predict the logarithm of the distribution coefficient, Log D7.4 from the compound structures. The distribution coefficient (D) considers the partitioning of all ionic species of a compound. In order to calculate the Log D7.4 value for a test compound, both the logarithms of the partition coefficient (Log P) and the acid dissociation constants (pKa) are needed for weak acids or bases. To perform these calculations, the test compound structure is drawn graphically, after which the computational program PrologD automatically calculates Log D7.4. The pKa of cationic lipids were also theoretically calculated using this software.
2.6 Forced lipid degradation preparation
Oxidation of Cationic Lipid 2 was performed by adding 60 μL 30% H2O2 to 5 mL of 85 mg/mL Cationic Lipid 2 in pure ethanol. This sample was stirred for 4 h before it was injected at 1 μL along with an untreated Cationic Lipid 2. PEG Lipid 2 hydrolysis was performed by treating 2 mL of 0.7 mg/mL PEG Lipid 2 solution in ethanol with 0.1 mL of 0.01 N NaOH.
3 Results and discussion
3.1 Sample preparation diluent selection
In order to analyze lipid components in mRNA-LNP vaccines, the diluent selection is critical. The diluent ought to be able to dissolve each lipid component and dissociate any interactions between lipids and encapsulated mRNA. This is especially true for lipids with cationic functional groups that have strong ionic interactions with negatively charged mRNA molecules.
The first diluent evaluated was a 1:1 Ethanol:DMSO mixture. A sample of 0.05 mg/mL mRNA loaded LNP containing PEG-DMG, Cholesterol, Cationic Lipid 1 and DSPC was analyzed using four different dilutions - four, six, eight, and ten (Figure 1). All four lipid components were adequately recovered at a sixfold, eightfold, and tenfold dilutions with recovery greater than 95%. However, after a four-fold dilution, both Cationic Lipid 1 and DSPC were severely under recovered at ≤35%. In addition, the %molar fraction of each lipid was consistent with the targeted formulation (±1%), except for the fourfold dilution sample. The four-fold sample showed an increased molar fraction for PEG-DMG and cholesterol, and a decreased molar fraction for cation lipid 1 and DSPC. Both the decrease in lipid recovery and a decrease in molar ratio suggest that the LNP and mRNA were either not fully soluble or the not fully dissociated from each other. A new diluent was considered to achieve greater solubility of all lipid and mRNA components at a lower dilution.
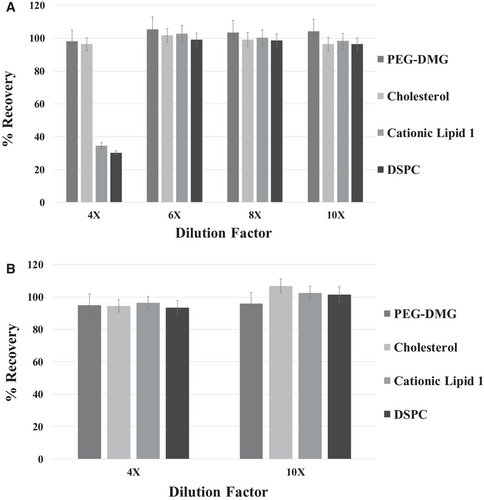
When optimizing the new diluent, a similar dielectric solvent strength as ethanol:DMSO (1:1) was desired while also increasing the amount or strength of a nonpolar solvent for adequate solubility of all lipids. Linear combinations using the dielectric constants of the pure solvents and their volume were used to estimate solvent combinations with similar dielectric solvent strength as ethanol: DMSO (1:1). A diluent with a combination of ethanol and formamide at a 85:15 ratio was chosen.
Another dilution experiment measuring an LNP sample containing 0.05 mg/ml mRNA was analyzed using dilution factors of 4 and 10. The ethanol: formamide diluent showed all lipid components had greater than 93% recovery and the target molar fraction was observed in both dilutions tested (Figure 1b). These data suggest that the ethanol:formamide diluent was able to disrupt all non-covalent interactions between the mRNA and lipids and maintain solubility for both mRNA and lipids during analysis. Based on this data, ethanol: formamide (85:15) was chosen as the optimal diluent for mRNA-loaded LNP vaccine samples. Furthermore, when evaluating samples with various mRNA concentrations, samples were diluted based on the concentration of mRNA due to a fixed mRNA to lipid ratio. For example, a 0.05 mg/mL mRNA must be diluted 4-fold and a 0.1 mg/mL mRNA sample 8-fold for full recovery of lipids and be within the linear range.
3.2 Chromatographic separation
A UHPLC method that can quantitate neutral and cationic lipids was developed for the evaluation of LNPs containing mRNA. The final chromatographic conditions consisted of a step gradient with two isocratic holds after various chromatographic conditions were explored during method development.
Potential degradants such as myristic acid, lyso-PC 1 and 2, and stearic acid are more hydrophilic than their parent lipids and eluted in the first isocratic hold. The four main lipid components are highly hydrophobic and eluted in the second isocratic hold. Two isocratic holds were chosen to minimize the effect of each analytes response factor based on mobile phase composition [20]. Impurities or degradants were monitored by loss of four main lipid peaks and percent impurity analysis for any addition of new, unspecified peaks. Percent impurity analysis was performed by integrating any peaks apart from the void, system, and parent lipid peaks. This analysis was monitored and compared in stability studies to the zero time point. As previously mentioned, based on the relative response of each analyte, the universal response of both parent lipids and their degradants are not achievable with this method. It would be worth exploring this method with the use of an inverse gradient or another means to a more universal response for impurity analysis utilizing a CAD.
Figure 2A shows the separation of an mRNA-LNP sample containing PEG-DMG, Cholesterol, Cationic Lipid 1, and DSPC. These lipid components are separated in order of increasing retention time 9.60, 10.15, 12.00, and 13.40 min. Figure 2B illustrates the separation of an mRNA-LNP sample containing the four lipids PEG Lipid 2, Cholesterol, Cationic Lipid 2, and DSPC in order of increasing retention time at 9.25, 10.15, 11.75, and 13.40 min. In both formulations, the four main lipids were baseline resolved for identification and content analysis.
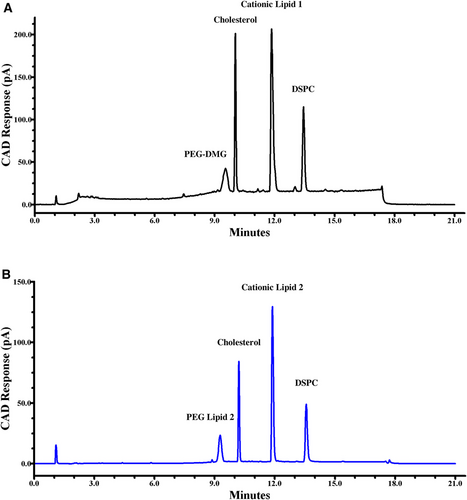
Of the four lipid analytes, three of them (PEG-DMG, Cholesterol, and DSPC) have an overall neutral charge and are not affected by mobile phase pH. These neutral and zwitterionic lipids were well separated prior to introducing a mobile phase pH modifier. Each analyte's calculated LogD7.4 values helped predict their retention under reversed phased conditions, that is, retention increases with an increase in LogD7.4 value. DSPC has the highest calculated LogD7.4 value of 13.60 of the neutral lipids and as predicted eluted after cholesterol and both PEG-conjugates. Cholesterol's calculated value was 9.85 and eluted well before DSPC. The predicted LogD7.4 value for the neutral and zwitterionic lipids correlated well with their retention times. Neither PEG Lipid 2 or PEG-DMG LogD7.4 values were predicted for this study due to the complexity of the polymer. It is conceivable that polyethyleneglycol's (PEG) large, hydrophilic head group plays an important role in reducing its hydrophobicity and retention in this method. Cationic Lipid 1 and Cationic Lipid 2's LogD7.4 values were theoretically calculated to be 15.48 and 12.54, respectively. These values were somewhat helpful in predicting the strength of their hydrophobicity. Instead, their retention was greatly influenced from their ionizable amine funtional group.
The mobile phase pH was a crucial attribute that influenced the retention of the cationic lipids by controlling protonation of their amine functional groups. To start, triethylamine was chosen due to its volatility and its ability to prevent secondary interactions between the amine functional groups and silica base stationary phases thereby reducing tailing of the cationic lipid peaks (data not shown) [21, 22].
When studying LNP samples containing Cationic Lipid 1, the mobile phase pH was adjusted to 3.5 by mixing formic acid at a 5:2 ratio with triethylamine – purchasable from Sigma Aldrich. Optimizing the pH to 3.5 controlled the elution of Cationic Lipid 1, with a theoretical pKa of 9.37 - between cholesterol and DSPC (Figure 2A). Doing so provided sufficient resolution for impurity and degradant analysis.
When analyzing LNP samples containing Cationic Lipid 2 at pH 3.5, it eluted prior to cholesterol. The Cationic lipid 2, with a theoretical pKa of 9.70, eluted between PEG Lipid 2 and cholesterol, further demonstrating the effect of mobile phase pH on cationic lipid separation (Figure 3A). The separation between Cationic Lipid 2 and PEG Lipid 2 was not optimal for quantitation or impurity analysis. So, a mobile phase pH of 3.5 was not used for analyzing Cationic Lipid 2, and a higher mobile phase pH was investigated.
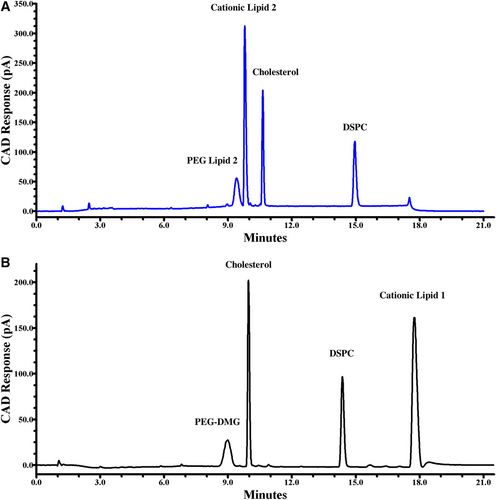
To increase the pH above 3.5, acetic acid was substituted for formic acid. This substitution, after pH adjusting to 5.4, eluted Cationic Lipid 2 between cholesterol and DSPC providing comparable retentions as with the more acidic mobile phase. Similarly, a retention time shift was observed for Cationic Lipid 1 when using a pH 5.4 mobile phase (Figure 3B). At higher pH, there was less ionization of the amine functional group resulting in a more hydrophobic molecule. Consequently, causing Cationic Lipid 1 to elute after DSPC.
3.3 Forced lipid degradation study
This method was optimized to capture potential degradation products of the four lipids measured in each method. An impurity reference standard was prepared using myristic acid, Lyso-PC1, Lyso-PC2, stearic acid, and a known degradant of Cationic Lipid 1 dissolved in pure ethanol. Myristic acid was used because it is a hydrolysis degradant of both PEG lipids. Lyso-PC1, Lyso-PC2, and stearic acid were chosen because they are hydrolysis degradants of DSPC. Cholesterol is known to be stable and therefore no suspected degradants of cholesterol were not used in this experiment [23].
Another degradant is the oxidation product of Cationic Lipid 2 that eluted between cholesterol and Cationic Lipid 2 at 10.6 min (Figure 4B) [24]. Forced degradation products of PEG Lipid 2 were determined by hydrolysis under basic conditions. Three degradation peaks were observed, see Figure 4C. Though mRNA-LNP vaccines would not likely be exposed to NaOH or high concentrations of H202, these conditions were chosen in order to evaluate chromatographic selectivity of the analytes’ degradants, especially when these samples were monitored for stability over 12 months.
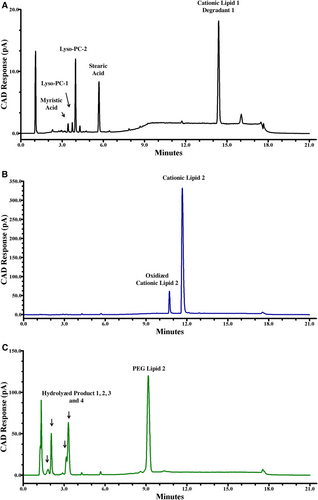
Figure 4A features a chromatogram of the lipid hydrolysis degradants, myristic acid, lyso-PC1, lyso-PC2, and stearic acid. All were resolved in order of increasing retention times 3.5, 3.8, 4.0, and 7.5 min in the first isocratic hold of the gradient at 79% mobile phase B. From 5.0 min to 7.50 min, mobile phase B is ramped to 98% to elute the four main lipid components, Cationic Lipid 2 oxidation, and the known degradant of Cationic Lipid 1.
3.4 Method validation
The method's accuracy, repeatability (intra-assay precision), inter-assay precision, specificity, linearity, and range were all validated for this UHPLC-CAD method. Validation results are summarized in Table 1.
| PEG-DMG* | PEG Lipid 2** | Cholesterol* | Cationic Lipid 1* | Cationic Lipid 2** | DSPC* | |
|---|---|---|---|---|---|---|
| Accuracy (%Recovery) | 93–96% | 98–102% | 99–103% | 95–97% | 98–102% | 96–99% |
| Intra-assay precision (%RSD) | 1–7% | 1–3% | 1–3% | 1–6% | 1–3% | 1–3% |
| Inter-assay precision (%RSD) | 5–6% | 1% | 1–2% | 5–6% | 1% | 1–2% |
| Linearity (R2) | 0.993–0.997 | 1.000 | 1.000 | 0.993–0.999 | 1.000 | 0.999 |
| Linear range (mg/mL)*** | 0.007–0.019 | 0.017–0.027 | 0.036–0.056 | 0.067–0.163 | 0.086–0.130 | 0.025–0.038 |
- * Mobile Phase Modifier: FA/TEA
- ** Mobile Phase Modifier: AA/TEA
- *** Lower limit of linear range indicates qualified LOQ of each lipid component. LOD was not determined.
The accuracy of this method was determined by spike recovery using a spike of 20%, 50%, and 70% of each lipid. The intra-assay precision of six injections was between 1 and 7% for all lipids (n = 6). Inter-assay precision was determined by evaluating the same sample between three separate runs on three different days. The inter-assay precision results for all lipids were between 1% and 6% (n = 3).
Four independent preparations of a negative control sample were prepared, and each preparation was injected once. The negative control sample consisted of the formulation buffer without lipids or mRNA. The negative control chromatogram and blank chromatogram were compared. Peaks with the same retention time in the blank were disregarded when compared to the negative control to obtain the peaks that were only related to the negative control. No interfering peaks from the negative control sample were observed.
The previously mentioned oxidation study of Cationic Lipid 2 was performed to demonstrate specificity for this method. Linearity was evaluated using a quadratic fitting between three independent runs using the concentration ranges listed in Table 1. The correlation coefficient (R2) of each quadratic fit was 0.993–1.000 for all lipids.
Assay performance was monitored using a control chart and a positive control sample. The performance was monitored over the course of 28 months with an mRNA-LNP sample containing Cationic lipid 1, and 15 months with an mRNA-LNP sample containing Cationic Lipid 2. Their results are listed in Table 2. All lipid concentration measurements had a % RSD of < 5% with the exception of PEG-DMG and PEG Lipid 2. Both PEG-lipid conjugates had a % RSD <10%. This is likely because each of the two PEG-conjugate lipids is the lowest concentrated relative to the other lipids. Their broad peak shape also likely contributes to this variability.
| mRNA Loaded LNP Control (n = 80) | ||||
|---|---|---|---|---|
| PEG DMG | Cholesterol | Cationic Lipid 1 | DSPC | |
| %RSD | 7.1 | 4.1 | 4.7 | 4.0 |
| mRNA Loaded LNP Control (n = 95) | ||||
| PEG Lipid 2 | Cholesterol | Cationic Lipid 2 | DSPC | |
| %RSD | 5.8 | 3.5 | 4.1 | 3.5 |
Finally, the LOQ of this method is listed as the lowest range of lipids in Table 1. LOD was not determined in this paper.
3.5 Lipid stability
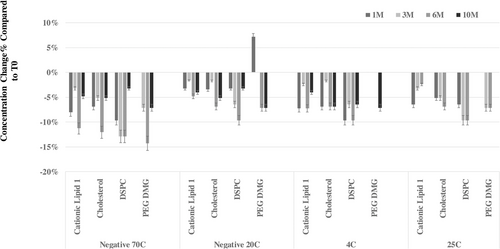
This lipid assay was used to monitor lipid stability at four temperatures –70°C, –20°C, 4°C, and 25°C and wsd pulled for analysis at the following time points: initial (time zero), 1 month, 3 months, 6 months, and 10 months. The stability data are illustrated in Figure 5 for the following four main lipids: PEG-DMG, cholesterol, Cationic Lipid 1, and DSPC. It is a relative comparison of each time point with the initial –70°C time zero measurement. Each sample from each timepoint was prepared in duplicate, and each timepoint sample submission was tested once. PEG-DMG showed 0% change at –70°C, 4°C, and 25°C in 1 M, –20°C, and 4°C in 3 M and 4°C in 6 M. The stability study at elevated temperature, 25°C, was run for 6 months and discontinued due to significant mRNA degradation. The decrease of the lipid concentration was approximately 10–15% at 6-month time point under –70°C storage temperature, which was the most significant change among all the time points at all the temperatures. The trend of the stability data across the temperatures indicates it may be due to sample handling, variability from sample pull date to time of testing, or assay variability instead of chemical degradation. Samples stored at higher temperatures for longer times would be expected to show more lipid concentration decrease if it was caused by chemical degradations, which was not observed in the stability study. Therefore, the stability results demonstrated all four lipids were chemically stable in the mRNA vaccine product at all the evaluated temperatures.
4 Concluding remarks
An efficient and sensitive reverse-phase UHPLC-CAD method was developed for the analysis of lipid components of mRNA encapsulated lipid nanoparticles (LNPs) in order to support process and formulation development studies and clinical material release and stability testing. The sample preparation and chromatographic conditions were optimized to fully recover and separate the main lipids and their potential degradants. This method was validated to be linear, precise, accurate, and specific for two LNP formulations: PEG-DMG, cholesterol, Cationic Lipid 1 and DSPC, and PEG Lipid 2, Cholesterol, Cationic Lipid 2, and DSPC.
This robust separation makes the method ideal for future work to support mRNA-LNP vaccines or other LNP containing drug candidate development for measuring lipid content and identity as well as monitoring the stability of LNP formulations. With optimization of the mobile phase pH other amine-containing lipids can be controlled to elute in-between cholesterol and DSPC and main reasonable run times. We think this method will be a beneficial contribution to the growing interest LNP technologies – not only in vaccines, but also therapeutics and there drug candidates utilizing this technology.
Acknowledgments
We would like to extend our gratitude to Dr Adam Socia and Dr Ping Zhuang for providing critical review to the manuscript. We also would like to extend our gratitude to our colleagues at Merck & Co., Inc., Kenilworth, NJ, USA Vaccine Process Development and Vaccine Drug Product Development MRL for providing us with all of the LNP materials used for this study.
The authors have declared no conflict of interest.
Open Research
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.