Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems
Abstract
Conventional chemotherapeutic approaches in cancer therapy such as surgery, chemotherapy, and radiotherapy have several disadvantages due to their nontargeted distributions in the whole body. On the other hand, nanoparticles (NPs) based therapies are remarkably progressing to solve several limitations of conventional drug delivery systems (DDSs) including nonspecific biodistribution and targeting, poor water solubility, weak bioavailability and biodegradability, low pharmacokinetic properties, and so forth. The enhanced permeability and retention effect escape from P-glycoprotein trap in cancer cells as a passive targeting mechanism, and active targeting strategies are also other most important advantages of NPs in cancer diagnosis and therapy. Folic acid (FA) is one of the biologic molecules which has been targeted overexpressed-folic acid receptor (FR) on the surface of cancer cells. Therefore, conjugation of FA to NPs most easily enhances the FR-mediated targeting delivery of therapeutic agents. Here, the recent works in FA which have been decorated NPs-based DDSs are discussed and cancer therapy potency of these NPs in clinical trials are presented.
Abbreviations
-
- 5-FU
-
- 5-fluorouracil
-
- BO
-
- borneol
-
- BSA
-
- bovine serum albumin
-
- CAP
-
- capsaicin
-
- CS
-
- chitosan
-
- CT
-
- computed tomography
-
- Cur
-
- curcumin
-
- CD/5-FC
-
- cytosine deaminase/5-fluorocytosine
-
- DDS
-
- drug delivery system
-
- DEX
-
- dextran
-
- DOX
-
- doxorubicin
-
- DTX
-
- docetaxel
-
- EPR
-
- enhanced permeability and retention
-
- ETB
-
- erlotinib
-
- FA
-
- folic acid
-
- FR
-
- folic acid receptor
-
- GNP
-
- gold nanoparticle
-
- GO
-
- graphene oxide
-
- HA
-
- hyaluronic acid
-
- LD
-
- monolayer lipid
-
- Lf
-
- lactoferrin
-
- LS
-
- liposome
-
- MNP
-
- magnetic nanoparticle
-
- MRI
-
- magnetic resonance imaging
-
- MTN
-
- mitoxantrone
-
- MTX
-
- methotrexate
-
- NIR
-
- near-infrared
-
- NP
-
- nanoparticle
-
- OA
-
- oleic acid
-
- OQLCS
-
- octadecyl-quaternized lysine modified chitosan
-
- PAMAM
-
- polyamidoamine
-
- PDT
-
- photodynamic therapy
-
- PEG
-
- polyethylene glycol
-
- PEI
-
- polyethyleneimine
-
- PHis
-
- poly(l-histidine)
-
- PLA
-
- polylactic acid
-
- PLGA
-
- poly(lactide-co-glycolide)
-
- PLLA
-
- poly(l-lactide)-b-poly(ethylene glycol)
-
- PLS
-
- polymeric liposome
-
- PNIPAAm-co-OA
-
- N-isopropylacrylamide-oleic acid
-
- PTX
-
- paclitaxel
-
- QU
-
- quercetin
-
- RSV
-
- resveratrol
-
- siVEGFA
-
- siRNA against vascular endothelial growth factor A
-
- Tf
-
- transferrin
-
- UCL
-
- upconversion luminescence
-
- UCNP
-
- upconverting nanoparticle
1 INTRODUCTION
Nowadays nanoparticles (NPs) based technologies are developed in wide spectrum of prognosis, diagnosis, treatment, and prevention in medicine. These technologies are related with practical applications of different NPs including nano-polymers, nanofibers, nanomembranes, nanosized chips, and nanomachines for protein, nucleic acid, peptide, and therapeutic agent delivery or their applications in nanosensors-based detection (Petros & De Simone, 2010; Barratt, 2000; Narmani, Kamali, Amini, et al., 2018). The monodispersity, nanosize and shape, good biocompatibility, high capacity of surface modification and functionalization, great in vivo stability, excellent pharmacokinetic properties, and other specific aspects of NPs indicate great promise for their application in the treatment of cancers (Hawker & Wooley, 2005; Kesharwani, Jain, & Jain, 2014). These various physicochemical and biocompatible features of NPs have made them as potent therapeutic vehicle for cancer therapy. Furthermore, NPs have been led to increase the circulation half-life of therapeutics and improve their tumors accumulation in body (Jadidi-Niaragh et al., 2017).
On the other hand, conventional therapeutic anticancer agents, due to their nontargeted distributions in the whole body, are typically affected both normal and cancer cells. This general drug distribution decreases the effective cytotoxic dose effect of anticancer on cancerous cell and mostly indicates the growth inhibitory effect on normal cells (Allen, 2002; Morgillo & Lee, 2005). This low dose effect on cancer cells is also eliminated via multidrug resistance process in these cells and thereby, the remarkable toxic effects of anticancer agents are threated the normal cells. Moreover, the intrusive processes in cancer therapy such as surgery to remove the tumor that has been followed by chemotherapy radiation, are accompanied with extreme ailment in patient and lead to organ amputation and even patient death in some cases (Stein & Skinner, 2006). In order to overcome these nontargeted and invasive surgeries approaches, the targeted (active) drug delivery is so crucial to affect cancer therapy. The active targeting strategies can increase the intracellular concentration of therapeutic agents in cancer cells while prevent the cytotoxic effects in normal cells (Figure 1).
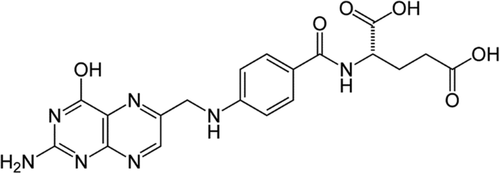
Folic acid (FA; Figure 2; Mr ∼38 kDa) is one of the cancer cell-targeted biomolecules with high overexpressed receptor to avoid formidable membrane barrier of tumors with poorly formed vasculature (Amini et al., 2019; Yoo & Park, 2004). As a kind of vitamin, FA is essential for the biosynthesis of nucleotide bases and cell proliferation. The physiological FA is transported using the cell membrane-associated proteins or folic acid receptor (FR) via receptor-mediated endocytosis (Turk et al., 2002). FR has overexpression in various human carcinomas including breast, ovary, kidney, lung, and so forth, and it is considered as a tumor-specific target platform due to its accessibility to intravenous drugs (Toffoli et al., 1997; Zhong et al., 2017). FR-α and FR-β as glycosylphosphatidylinositol-anchored membrane glycoproteins are common overexpressed receptors on the surface of cancer cells. In comparison to FR-β, high affinity for the circulating folic acid coenzyme (6S)-5-methyltetrahydrofolic acid and physiologic FA are major properties of FR-α (Da Costa & Rothenberg, 1996; Narmani, Mohammadnejad, & Yavari, 2019). Other isoforms of FR are FR-γ and FR-γ′ that are soluble forms of the human FR specific for hematopoietic tissues and lymphoid cells. The affinities of these receptors are KD ∼0.1 , ∼1, and ∼0.4 nM for FR-α, FR-β, and FR-γ, respectively (Bueno, Appasani, Mercer, Lester, & Sugarbaker, 2001; Shen, Wu, Ross, Miller, & Ratnam, 1995; Wu, Gunning, & Ratnam, 1999). Also, there is a remarkable correlation between the degree of FR-α expression in cancer cells and their resistance to standard chemotherapy. As high level of FR expression in cancers tissue has been directly related to survive of cancer cells from standard chemotherapy (Toffoli et al., 1998). FR-β expression level is elevated in most of nonepithelial origin malignancies such as myelogenous leukemias and sarcomas (Reddy et al., 1999). On the other hand, FR-γ and FR-γ′ expression high levels are also related to certain hematopoietic malignancies (Ross et al., 1999). Thus, cancer selectivity of FA and FA conjugates can be considered as potent approach in NP-based targeted delivery of anticancer therapeutic agents in cancer diagnosis and therapy (Figure 3; Narmani, Yavari, & Mohammadnejad, 2017).
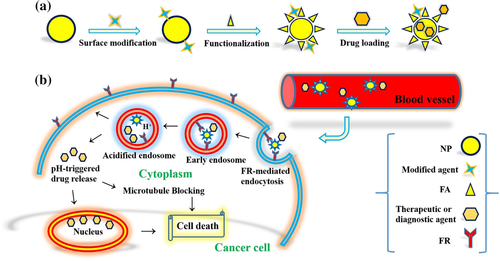
There are six FA-linked practical applications for targeting delivery of therapeutic agents that have been tested in the clinic until now (Leamon et al., 2007). These practical cases are including, FA-targeted protein toxins (such as use of FA-Pseudomonas exotoxin conjugate to kill FR+ cancer cells), FA-targeted chemotherapeutic agents (such as FA-paclitaxel and FA-camptothecin in order to tumor inhibition), FA-targeted immunotherapy (such as use of FA/anti-T cell receptor to educate the immune system to recognize tumor activating mutations), FA-targeted gene therapy (such as FA-nonviral gene therapy vectors), FA-targeted antisense oligodeoxyribonucleotides (ODN) and small interfering RNAs (siRNA) delivery (such as use of ODN or siRNA to knockout or modulate gene expression), and design of FA-targeted NPs in targeted cancer therapy (Leamon et al., 2007; Tranoy-Opalinski, Fernandes, Thomas, Gesson, & Papot, 2008).
On the other hand, use of new and innovative protocols and methods for the synthesis and functionalization of NPs has made excellent scientific and clinic community between molecular-targeted drug delivery and commercialization (Shi, Kantoff, Wooster, & Farokhzad, 2017). As, many more formulations of targeted NPs are annually progressing to clinical trials investigation (Phase I–IV) that liposomal and polymeric formulations represent the biggest share of the targeted NPs under clinical evaluation. Even several formulations of liposomal and polymeric NPs earned the FDA-approved and introduced in the market (Shi et al., 2017; Svenson, 2012). However, there are several key obstacles and challenges which are related to the clinical and translational development of targeted NPs in nanomedicine, such as lack of clear regulatory guidelines that are specific to targeted NPs, limited understanding of the biological stability of targeted NPs in human body and serum, and limited knowledge about NPs biocompatibility and biodegradability (Hua, De Matos, Metselaar, & Storm, 2018; Svenson, 2012). On the other hand, there is some incoordination between members of the investment community in conceptual understanding of the nanomedicine and scientific research recognize that has very low understanding about business expertise to develop commercial product in nanomedicine.
Also, there are other studies that previously evaluated some other properties of FA-functionalized NPs as delivery systems. In a work by Bahrami et al. (2015), the general properties of FA-functionalized NPs and their delivery behavior in preclinical experiments evaluated. Also, their group just prepared some of the information about specific types of NPs without complete classification and preparation methods of nanocomplexes. However, in this work, most of the NPs classified first and then different properties and synthetic methods of nanocomplexes were reviewed in detail. In this work, many angels of FA-functionalized NPs and their practical applications in preclinical cases were also evaluated. Also, this study focused on recent studies. In another study by Samadian, Hosseini-Nami, Kamrava, Ghaznavi, and Shakeri-Zadeh (2016), some of the specific aspects of FA-functionalized gold NPs and their applications in therapeutic and diagnostic delivery systems were collected. However, this work is completely different from their work because in this study, different types of FA-functionalized NPs (not just gold NPs) were investigated and different characteristics of different NPs were reviewed.
Several NPs, as drug delivery systems (DDSs), are the most important applications in cancer prognosis, diagnosis, and therapy (Misra, Acharya, & Sahoo, 2010; Rawat, Singh, & Saraf, 2006). These practical NPs are including polymer-based (biopolymers and chemopolymers), lipid-based (liposomes and micelles), oxide-based (iron oxide, graphene oxide, etc.), and upconversion NPs (Tables 1 and 2). These NPs, due to their specific properties, are excellent nanocarriers as long as implement such intelligence vehicle in cancer targeting that is possible via their surface functionalization with FA as cancer-targeted agent. In the following summary of significant applications of FA-decorated NPs in active delivery of therapeutic agents and their importance will state.
| Nanocarriers | Bioactive agents | Characteristics | Comments | Kind of cell lines and tumors | References |
|---|---|---|---|---|---|
| Bio-polymers | |||||
| FA-SOCS NPs | HCPT | Core-shell micelle for active delivery of hydrophobic agent | In vitro and in vivo | Bel-7402 hepatoma cell line and Bel-7402 tumor-bearing nude mice | Zhu, Cao, Cui, Qian, and Gu (2013) |
| FA-(PNIPAAm-co-OA)-g-CS NPs | ETB | Self-assembled core-shell micelle | In vitro | OVCAR-3 ovarian carcinoma and A549 human non-small-cell lung cancer cell lines | Fathi et al. (2017) |
| FA-PLA-PEG NPs | DOX | Hybrid core-shell micelle for active drug delivery | In vitro | SKOV3 ovarian cancer cell line | Hami, Amini, Ghazi-Khansari, Rezayat, and Gilani (2014) |
| FA-PLLA-PEG NPs | DOX | Self-assembled core-shell micelle for codelivery | In vitro | A2780 ovarian cancer cell line | Kim, Lee, Oh, Gao, and Bae (2008) |
| FA-Au-PLA-PEG NPs | DOX | Self-assembled micelle for active delivery of hydrophobic agent | In vitro | 4T1 breast cancer cell line | Prabaharan, Grailer, Pilla, Steeber, and Gong (2009) |
| FA-PLGA-PLS NPs | DOX, pEGFP | Self-assembled core-shell micelle for codelivery | In vitro | MDA-MB-231 breast cancer cell line | (Wang, Zhao, Su, et al., 2010) |
| FA-PLGA-Lf NPs | Etoposide | Co-targeted delivery | In vitro | U87MG glioma cells cell line | Kuo and Chen (2015) |
| FA-PLGA-CS NPs | Ins | Globular electrostatic self-assemble | In vitro and in vivo | HT-29 colon cancer cell line and diabetic rats | (Xu, Jiang, Yu, et al., 2017) |
| FA-PEG-PLLA NPs | Plasmid DNA | Three-layered co-polymeric micelle | In vitro and in vivo | RAW264.7 macrophage cell line and mice model | Mohammadi et al. (2016) |
| FA-PEG–PLA NPs | Cur | Amphiphilic copolymeric nanocarrier | In vitro | MCF-7 breast cancer and HepG2 liver cancer cell lines | (Yang, Chen, Zhao, et al., 2014) |
| FA-DEX-BSA NPs | DOX | Self-assembled core-shell micelle | In vitro and in vivo | H22 hepatoma cell line and murine ascites hepatoma H22 tumor-bearing mice | (Hao, Ma, Huang, He, & Yao, 2013) |
| FA-DEX NPs | RSV | Core-shell micelle | In vitro | A549 human non-small-cell lung cancer cell line | Zhao et al. (2017) |
| FA-HA-PHis NPs | DOX | pH sensitive self-assemble micelle | In vitro | MCF-7 breast cancer cell line | Qiu et al. (2014) |
| FA-HA NPs | PTX | Dual-targeted self-assemble micelle | In vitro | MCF-7 breast cancer cell line | Liu et al. (2011) |
| Chemo-polymers | |||||
| FA-PAMAM/BO NPs | DOX | Dual-targeted delivery | In vitro and in vivo | C6 glioma cell line and glioma-bearing rats | Xu et al. (2016) |
| FA-PAMAM-PEG-99mTc NPs | 5-FU | Theranostic targeted codelivery | In vitro and in vivo | MCF-7 breast cancer and C2C12 cell lines and 4T1 tumor bearing BALB/C mice | Narmani et al. (2017) |
| FA-PAMAM NPs | siVEGFA | Localized FR-α mediated gene delivery | In vitro and in vivo | HN12 and HN12-YFP cell lines and athymic nude mice | (Xu, Yeudall, & Yang, 2017) |
| FA-PEG-b-(ɛCL-g-PEI)-b-ɛCL NPs | DOX, P-gp siRNA | Amphiphilic core-shell polymer-based micelle for codelivery | In vitro and in vivo | MCF-7 breast cancer and MCF 7/ADR cell lines and athymic nude mice | Wu et al. (2016) |
| FA-C60-PEI NPs | DTX | Amphiphilic core-shell micelle | In vitro and in vivo | PC3 prostate cancer cell line and S180 prostate tumor models | Shi et al. (2013) |
| FA-PEI-OA NPs | LOR-2501 | Amphiphilic carriers for targeted delivery | In vitro | HeLa cervical cancer and SK-HEP-1 human cell lines | Yang et al. (2015) |
| FA-PEG-PEI NPs | pCD/5-FC, pTRAIL | Hydrophilic globular carrier for delivery | In vitro and in vivo | C6 glioma cell line and glioma-bearing rats | Liang et al. (2009) |
- Abbreviations: 5-FU, 5-fluorouracil; BO, borneol; BSA, bovine serum albumin; CS, chitosan; Cur, curcumin; DEX, dextran; DOX, doxorubicin; ETB, erlotinib; FA-SOCS, FA-fuctionalized N-succinyl-N′-octyl CS; HA, hyaluronic acid; HCPT, hydroxycamptothecin; Lf, lactoferrin; NP, nanoparticle; OA, oleic acid; PAMAM, polyamidoamine; PEG, polyethylene glycol; PEI, polyethyleneimine; PHis, poly(l-histidine); PLA, polylactic acid; PLGA, poly(lactide-co-glycolide); PLLA, poly(l-lactide)-b-poly(ethylene glycol); PLS, polymeric liposome; PNIPAAm-co-OA, N-isopropylacrylamide-oleic acid; PTX, paclitaxel; RSV, resveratrol.
| Nanocarriers | Bioactive agents | Characteristics | Comments | Kind of cell lines and tumors | References |
|---|---|---|---|---|---|
| Bilayer lipid NPs | |||||
| FA-LS-Tf NPs | DOX | Dual-targeted LS for targeted delivery | In vitro and in vivo | HeLa cervical and A2780-ADR ovarian cell lines and athymic nude mice | Sriraman, Salzano, Sarisozen, and Torchilin (2016) |
| FA-LS-PEG NPs | CAP | Amphiphilic globular LS for targeted delivery | In vitro and in vivo | SKOV-3 ovarian cell line ovarian tumor bearing rats | Lv et al. (2017) |
| FA-PEG-LS-PLGA NPs | DTX | Amphiphilic functionalized-LS for targeted delivery | In vitro | MCF7 breast cancer and NIH/3T3 fibroblast cell lines | Liu, Li, Pan, Liu, and Feng (2010) |
| Monolayer lipid NPs | |||||
| FA-PEG-LD-PLGA NPs | PTX | Self-assembled core-shell micelles | In vitro and in vivo | HeLa cervical and A549 human non-small-cell lung cancer cell lines and cervical tumor bearing SCID mice | Zhao et al. (2012) |
| FA-LD-PLGA NPs | MTX | Self-assembled amphiphilic hybrid micelles | In vitro | MDA-MB-231 breast cancer, PC3 prostate, and HT29 colon cell lines | Tahir et al. (2017) |
| FA-LD-FA NPs | MTN | Self-assembled amphiphilic block or graft copolymers | In vitro | HeLa cervical cancer cell line | Chen et al. (2013) |
| FA-PEG-PPO-PEG NPs | DOX, QU | Amphiphilic core-shell micelles for targeted codelivery | In vitro | HeLa cervical cancer cell line | Hassanzadeh, Feng, Pettersson, and Hakkarainen (2015) |
| Iron oxide NPs | |||||
| FA-DEX-MNPs | DOX | Hydrophilic biocompatible core-shell NPs for dual-targeted imaging and therapy | In vitro | MCF-7 and MDA-MB-468 breast cancer cell lines | Varshosaz, Sadeghi-Aliabadi, Ghasemi, and Behdadfar (2013) |
| FA-BSA-MNPs | DOX | Hydrophilic core-shell NPs for active therapy | In vitro and in vivo | KB human nasopharyngeal epidermal carcinoma cell line and KB tumor bearing BALB/c nude mice | (Yang, An, Miao, et al., 2014) |
| FA-PEG-FITC-PEI-MNPs | Fe3O4 | Hydrophilic core-shell NPs for MRI | In vitro and in vivo | KB human nasopharyngeal epidermal carcinoma cell line and KB tumor bearing BALB/c nude mice | Li et al. (2013) |
| FA-PEG-PEI-MNPs | DOX, Fe3O4 | Co-targeted hydrophilic core-shell NPs for codelivery | In vitro and in vivo | MCF7 breast cancer cell line and MCF7 tumor bearing cell BABB/c mice | Huang, Mao, Zhang, and Zhao (2017) |
| FA-S/ZnO-MNPs | Cur, Fe3O4 | Hydrophilic core-shell NPs for codelivery | In vitro | HepG2 liver cancer and MCF7 breast cancer cell lines | Saikia, Das, Ramteke, and Maji (2017) |
| FA-PEG-CMNPs | Fe3O4 | Dual-targeted core-shell NPs magnetic hyperthermia | In vitro | Normal human fibroblasts and HeLa cervical cancer cell lines | Sadhasivam, Savitha, Wu, Lin, and Stobinski (2015) |
| Graphene oxide NPs | |||||
| FA-BSA-GO NPs | DOX | Nano-hybrid sheets for targeted delivery | In vitro | MCF-7 human breast cancer and A549 human non-small-cell lung cancer cell lines | Ma et al. (2017) |
| FA-MNP-GO NPs | DOX | Dual-targeted core-shell NPs for delivery | _ | _ | (Wang, Zhou, Xia, et al., 2013) |
| FA-PVP-GO NPs | DOX | Targeted chemo-photothermal therapy | In vitro | HeLa cervical cancer and A549 human non-small-cell lung cancer cell lines | Qin et al. (2013) |
| Gold NPs | |||||
| FA-GNPs | DOX | Targeted NPs for imaging and therapy | In vitro | Human dermal fibroblasts (HDF) and HepG2 human liver cancer cell lines | Cheng, Gu, Cheng, and Wong (2013) |
| FA-PEG-siRNA-GNPs | si-RNA | Dual-targeted NPs for silencing and imaging | In vitro and in vivo | HeLa cervical cancer cell line and HeLa bearing nude mice | (Wang, Zheng, Peng, Shen, Shi, & Zhang, 2013) |
| FA-FITC-PEG-PEI-GNPs | Au2O3 | Targeted core-shell NPs for CT imaging | In vitro and in vivo | KB cell line and KB tumor-bearing BALB/c nude mice | Zhou et al. (2016) |
| FA-BSA-GNPs | Au2O3 | Targeted core-shell NPs for cancer cell detection | In vitro | HeLa cervical cancer cell line | Li, Cheng, Liu, and Chen (2016) |
| Upconversion NPs | |||||
| FA-Tm-UCNPs | Tm3+ | Targeted surface modified NPs for imaging | In vitro and in vivo | KB human nasopharyngeal epidermal carcinoma cell line and KB tumor-bearing BALB/c nude mice | Cao et al. (2011) |
| FA-Fe3O4-NaYF4:Yb/Er UCNPs | Yb3+, Er3+, Fe3O4 | Targeted core-shell NPs for theranostic codelivery | In vitro and in vivo | MCF-7 human breast cancer and HeLa cervical cancer cell lines and MCF-7 tumor-bearing nude mice | Zeng et al. (2015) |
| FA-SiO2-LaF3:Yb,Tm,Ho,Er UCNPs | Yb3+, Tm3+, Er3+, Ho3+ | Targeted core-shell NPs for dual-modality imaging of UCL and X-ray CT | In vitro and in vivo | MGC-803 and GES-1 human gastric cancer cell lines and MGC-803 tumor-bearing nude mice | Ma et al. (2012) |
| FA-PEG-NaYF4:Yb/Er UCNPs | Yb3+, Er3+, DOX | Targeted core-shell NPs for theranostic codelivery | In vitro | KB human nasopharyngeal epidermal carcinoma and HeLa cervical cancer cell lines | Wang, Cheng, and Liu (2011) |
- Abbreviations: BSA, bovine serum albumin; CAP, capsaicin; DEX, dextran; DOX, doxorubicin; DTX, docetaxel; FA, folic acid; GNP, GNP, gold nanoparticle; GO, graphene oxide; LD, monolayer lipid; LS, liposome; MTN, mitoxantrone; MTX, methotrexate; NP, nanoparticle; PEG, polyethylene glycol; PEI, polyethyleneimine; PLGA, poly(lactide-co-glycolide); PTX, paclitaxel; PVP, polyvinylpyrrolidone; QU, quercetin; Tf, transferrin; UCNP, upconverting nanoparticle.
2 POLYMER-BASED NPS IN DRUG DELIVERY
2.1 Biopolymers in drug delivery
2.1.1 Chitosan NPs
Targeted nanoscaled chitosan (CS)-based DDSs are the subject of interest as targeted carriers of therapeutics and diagnostics agents to cancer cells, which have improved systemic performance in cancer detection and therapy (Figure 4; Chen et al., 2008; Park et al., 2004). The CS, as natural biopolymer, has excellent biocompatibility, biodegradability, great bioavailability, hydrophilicity, and low toxicity, and it has also practical application in different forms including nanomicelles, NPs, microspheres, hydrogels, biofilms, and tablets (Rege, Garmise, & Block, 2003; Rezvani, Mohammadnejad, Narmani, & Bidaki, 2018). Furthermore, functional surface hydroxyl and amine groups facilitate their chemical modification to surface engineering in the CS backbone (Zhang, Ding, Yu, & Ping, 2007).
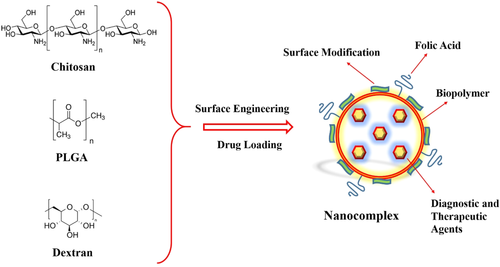
Also, CS as N-deacetylated derivative of chitin is a copolymer of glucosamine and N-acetyl-d-glucosamine. The micelle form of CS-based nanostructures (amphiphilic block copolymers) has shown broad spectrum of potency in anticancer agent delivery that are following by several research studies. In a work, Zhu, Cao, Cui, Qian, and Gu (2013) used the FA-functionalized N-succinyl-N′-octyl CS (FA-SOCS) micelle, as hydrophobic–hydrophilic core–shell, for targeted delivery system of 10-hydroxycamptothecin (HCPT) anticancer agent to targeted delivery. Average diameter size of these NPs was 122–145 nm. HCPT due to its insolubility in water, short half-life, and high toxicity to normal tissue cells needs to active delivery. Also, HCPT undergoes lactone ring-opening hydrolysis to inactive carboxylate form in physiological conditions and it is instable in body serum (Wang, Shang, Li, & Jiang 2009; Watanabe, Kawano, Toma, Hattori, & Maitani, 2008). Therefore, Zhu, Cao, Cui, Qian, and Gu (Zhu et al., 2013) applied the FA-targeted CS micelles to solve the water insolubility, provide sustained release and mainly improve the active delivery of this drug. Moreover, FITC-labeled FA-SOCS micelle remarkably evidenced the high internalization in in vitro and in vivo, while no HCPT signal was observed in free FA micelle uptake study. The outer hydrophilic layer of micelles is so crucial for improving the prolong circulation, escaping the immune clearance, and substantial accumulation in cancer site (Huo et al., 2010; Zhang, Huang, & Li, 2014). The pharmacokinetic behavior of the free HCPT, HCPT/SOC, and HCPT/folic acid-SOC micelles were investigated in the rats via I.V. administration. The total body clearance rates of free HCPT were about sevenfold faster than those HCPT/folic acid-SOC micelles, which have been shown translational development of targeted NPs in clinical applications. Also, plasma concentration rate of targeted micelles was more than four times in respect to nontargeted ones. On the other hand, tumor targeting potency of targeted micelles was investigated in Bel-7402 tumor-bearing mice by means of near infrared dye. As, targeted micelles were sharply detectable in the tumor site just 6 hr post-injection, it was 24 hr for nontargeted micelles. Moreover, it was revealed that NPs above 200 nm can be rapidly eliminated from the vascular pathway by macrophages and particles below 10 nm can be easily filtered by the kidney (Zhu et al., 2013).
In a research by Fathi et al. (2017), the maleic anhydride moieties were conjugated to hydroxyl sites of CS to produce the hydrophobic/hydrophilic nanomicelles. In order to this, sodium dodecyl sulfate/CS complex (SCCS) has been used for solubilizing CS in common organic solvents and dissolved in maleic anhydride to produce CS (O-maleoyl-SCCS) nanocomplex. Then, this organosoluble complex dissolved in N-isopropylacrylamide/oleic acid as hydrophilic/hydrophobic moieties to formation of N-isopropylacrylamide-oleic acid (PNIPAAm-co-OA)-g-CS micelles with free amino groups (mean particle size ∼100 nm). These intact amino groups are site of FA conjugation. Subsequently, erlotinib (ETB), as hydrophobic agent was physically encapsulated into micelles for treatment of metastatic advanced non-small cell lung and pancreatic cancers (Fathi et al., 2017). Eventually, it was concluded that the (Fluorescein isothiocyanate)-modified micelles can be delivered rapidly and effectively (more than six times internalization in comparison to nontargeted NPs) into the targeted cancer cells, significantly (Fathi et al., 2017). On the other hand, hemolysis assay was carried out to evaluate hemocompatibility of folic acid-modified micelles. As, the hemolytic ratio of the targeted micelles was less than 3%, that was in accordance with the guidelines of ISO/TR 7406 (Fathi et al., 2017).
In the amphiphilic polymers, hydrophobic core region serves as a reservoir for hydrophobic drugs, while hydrophobic core stabilization, polymers water-solubility of polymer is the fundamental role of hydrophilic shell (Adams, Lavasanifar, & Kwon, 2003). On the other hand, the various amphiphilic polymers can be formed micelles or self-aggregates nanocomplex in isotropic aqueous solution which is extended by intra- and intermolecular association between hydrophobic segments, primarily to minimize interfacial free energy (Mortensen, 2001). The rheological feature, small hydrodynamic radius, and thermodynamic stability are unique properties of polymeric micelles.
2.1.2 PLGA NPs and PLA NPs
Currently, the most important therapy approaches to circumvent side effects of chemotherapy and Multi drug resistance (MDR) are the use of biocompatible and biodegradable nano-biopolymers such as PLGA poly(lactide-co-glycolide), PLA (polylactic acid), and so forth in chemotherapeutic anticancer delivery (Figure 4; Hami et al., 2014; Kim et al., 2008). The PLGA is approved by FDA and it is classified safe for clinical use, as pharmaceutical excipients. Also, the great drug release behavior, excellent ability associate, and good ability of penetration the transmucosal are the main properties of PLGA NPs (Prabaharan et al., 2009; Zhou, Patel, Michael, Bertram, & Saltzman, 2012). On the other hand, the negative surface charge of PLGA makes it as suitable carrier in surface functionalization and drug conjugation and limits its interaction in normal cells.
In a research by (Wang, Zhao, Su, et al., 2010), the self-assemble cationic PLGA/FA which had been coated PEGlated polymeric liposome (PLS) core–shell NPs (FA-PLGA-PLS NPs) was developed to codelivery of pEGFP as a model of DNA and doxorubicin (DOX), as a model drug. So DOX was encapsulated and pEGFP was bound to the FA-PLGA-PLS NPs. In order to these syntheses, the cationic PEGlated amphiphilic octadecyl-quaternized lysine modified chitosan (PEG-OQLCS), cationic folic acid conjugated amphiphilic octadecyl-quarternized lysine modified chitosan (FA-OQLCS), and cholesterol (weight ratio 1/1/1, total lipids 30 mg) were dissolved in chloroform to create the organic phase. Cholesterol helps to maintain the structural stability of lipid shell. Then, chloroform was separated and thin film of lipid bilayer was obtained. Subsequently, the lipid film was dispersed in PLGA nanospheres solution and it was stirred to produce self-assemble micelles with maximum particle size ∼100 nm. The roles of PEG-OQLCS as protective coat and FA-OQLCS as targeted part are promoting the in vivo stability and monodispersity, and enhancing tumor accumulation and reducing the systemic toxicity, respectively (Wang, Zhao, Su, et al., 2010). Drug release (more than 50%) time of this NP was happened at first 6 hr. Codelivery efficiency of PLGA/FPL NPs was by 46.6%, while it was by 5.78 and 15.06% for free PLGA and FA-PLGA-PLS NPs, respectively, that it has been shown the FA-induced targeted delivery effects (Wang, Zhao, Su, et al., 2010). On the other hand, internalization efficiency was demonstrated about more than 50-fold for targeted micelles; as the bright red and green fluorescences were considerably observed in the cytoplasm around the nuclei which were indicated suitable codelivery of drug and gene. These results were demonstrated excellent potency of FA-based targeted therapy in clinical application.
In a report by Kuo and Chen (2015), the lactoferrin (Lf)- and FA-grafted PLGA NPs (Lf/FA/PLGA NPs; 181.9 ± 4.5 nm particle size with positive surface charge) were developed for targeted delivery of etoposide to inhibit the Glioblastoma (GBM) growth and penetrate in monolayer of human brain-microvascular endothelial cells (HBMECs). The Lf has been applied to permeate an in vitro blood–brain barrier (BBB) via receptor-mediated transcytosis (RMT) (Huang et al., 2007). Moreover, Lf can mediate the trigger the RMT pathway and accelerates the transportation of Lf/FA/PLGA NPs to HBMECs and makes a crucial role in delivering etoposide across the BBB by docking it. The sustained release was recorded for more than 50% of etoposide at first 7 days. On the other hand, the uptake of Lf/FA/PLGA NPs by U87MG cells was revealed the delivery of antitumor etoposide to brain cancer cells that was strongly related to FA (Kuo & Chen, 2015). Also, Lf could improve the uptake of Lf/FA/PLGA NPs by U87MG cells via binding to low-density lipoprotein receptor. Furthermore, the tiny NPs can be penetrate from BBB, however effective penetration was obtained via mediating specific blood proteins, including transferrin, leptin, insulin-like growth factors, and specifically Lf (Lf showed more effective penetration potency than others). Moreover, the other same work was carried out by (Xu, Jiang, Yu, et al., 2017) in order to active delivery of insulin in diabetic (as lethal disease) models. As specified, the subcutaneous needle injection of insulin is concomitant with lethal pain in patients. In order to overcome this disadvantage, FA-modified Ins-PLGA/CS NPs with 252.4 ± 4.6 nm in diameters were fabricated via electrostatic self-assembly approach by Xu et al. research work. The sustained release of insulin was about 35% at first 6 hr for globular positive-charge Ins-PLGA/FA-CS NPs. Furthermore, the serum which had been sustained release of insulin was significantly demonstrated that serum insulin level was maintained at 40 μIU/mL for long period time and subsequently it was decreased the percentage of initial blood glucose levels to half of maximum levels (100%; Xu, Jiang, Yu, et al., 2017). On the other hand, the enzyme inhibition test was performed to investigate the stability of insulin. Insulin is easy to damage in the presence of pepsin or pancreatin in gastrointestinal fluid. It was shown that about 10% of free insulin can be remained for 30 min in simulated gastric fluid after incubation and simulated intestinal fluid, while more than 80% of insulin can be remained after encapsulation of insulin into nanocarriers. Subsequently, many values of the insulin have been degraded for the free insulin by increasing the incubation time, while it was less than 40% for Ins-PLGA/FA-CS. Also, pharmacokinetics investigation of this micelle has been shown that oral delivery of insulin solution has no hypoglycemic effect, while blood glucose level has been reduced significantly after subcutaneous injection of 5 IU/kg insulin solution. Eventually, it was indicated that high hypoglycemic efficacy of the Ins-PLGA/FA-CS in the diabetic rats is related to the following features: the nanocomplex serves as an efficient tool for protection of insulin in acidify environment of the stomach, and the nanocomplex might increase the cellular permeability of the insulin and improve cellular uptake of Ins-PLGA/FA-CS nanocomplex (Xu, Jiang, Yu, et al., 2017).
In order to suppress the inappropriate activated macrophages, Mohammadi et al. (2016) were applied triblock copolymers FA-poly(l-lactide)-b-poly(ethylene glycol) (FA-PEG-PLLA), poly(l-lactide)-polyethylenimine-poly(l-lactide) (PLLA-PEI-PLLA), and PLLA-poly(ethylene glycol)-PLLA (PLLA-PEG-PLLA), as three-layered micelle to target FR-β that has high expression in this macrophages. FR-α has negligible accessibility to circulate folic acid-targeted drugs, because of its expression on the apical side of healthy tissues, while FR-β has great accessibility for activated macrophages. The three-layered micelle was optimized for the encapsulation of plasmid DNA at N/P ratio of 12. Subsequently, the transfection efficiency of micelle containing plasmid GFP-DNA was ascertained via isolation of spleen macrophages. The in vitro and in vivo FITC-labeled micelles high fluorescence intensity of FITC was quantified and a shift to higher fluorescence indicates the expression of FR in activated macrophages (more than 10 times in comparison to resting macrophages). Also, Green fluorescent protein (GFP) expression was shown more than three times to evaluate the transgene expression and results of gene expression in activated macrophages in comparison to resting macrophages. Gene release was determined with hydrogel retardation assay and no gene release was observed in physiologic pH condition, however about 15.3% DNA were released after 144 hr incubation in acidic pH (Mohammadi et al., 2016).
These amphiphilic micelles have excellent advantages such as good solubility of hydrophobic drugs, high loading capacity, great biocompatibility and biodegradability, reducing the nonspecific uptake by the reticuloendothelial system (RES), enhancing circulation time in blood, optimizing the anticancer efficiency of drug, overcoming the drug's drawbacks through minimizing its toxicity, overcoming the development of drug resistances, and siting specific targeting delivery (Hu et al., 2008; Ortiz et al., 2012; Xiao et al., 2012). In order to increase the drug's anti-angiogenic efficacy and enhance the antitumor efficacy, the curcumin-loaded copolymer FA-PEG-PLA, as amphiphilic micelles, were synthesized by (Yang, Chen, Zhao, et al., 2014). This nanocarrier was prepared by thin-film hydration method with mPEG2000-PLA2000 and Folate-PEG3000-PLA2000 (with 9:1 weight ratio) with average size of 70 nm. The drug loading and encapsulating efficiency of curcumin were by 4.84% ± 0.01% and 80.73% ± 0.16%. Moreover, the pharmacokinetic investigations in rats demonstrated that a three times enhancement in the half-life was achieved for Cur-loaded micelle formulations which were relative to solubilized Cur (Yang, Chen, Zhao, et al., 2014). Also, internalization studies were shown more than five times enhancement in cellular uptake.
2.1.3 Dextran NPs and hyaluronic acid NPs
Dextran (DEX), as biodegradable and biocompatible nanosize biomolecule, is a complex branched polysaccharide which has been made of many glucose molecules and has chains of varying lengths from 3 to 2,000 kD that straight chain consists of α-1,6 glycosidic linkages between glucose molecules (Figure 4). The saccharide-based nanocarriers are developed in order to improve the high anticancer efficacy, decrease the drug side effects, and prolong the drug circulation time in bloodstream, and so forth (Takara et al., 2012; Zhang et al., 2012). In a research by Hao, Ma, Huang, He, & Yao (2013), the bovine serum albumin-dextran-doxorubicin-FA (DOX/BSA-DEX-FA) conjugate was prepared by an esterification reaction between FA and dextran that fallowed by Maillard reaction between the DEX-FA and BSA to obtain BSA-DEX-FA with 90 nm in size. BSA has some advantages for NPs including reduced plasma protein adsorption on the particle surface, ease of NP purification, low cost, and unusual ligand-binding properties (Elzoghby, Samy, & Elgindy, 2012; Mohanta, Madras, & Patil, 2012). Also, BSA NPs were used in multilayer thin film via layer-by-layer self-assembly for targeting delivery. DOX loading amount and loading efficiency of the NPs are larger than 14 and 90%, respectively. Furthermore, drug release investigation was indicated more than fourfold sustained release versus free drug release. In this work, pH adjusting and heating process was applied to modulate the electrostatic and hydrophobic interactions between DOX and BSA, induce the self-aggregation of DOX and the denaturation/aggregation/gelation of BSA. On the other hand, the in vivo antitumor results were shown about three times survivability in DOX/BSA-DEX-FA treated animal models which were relative to nontargeted sample treatments (Hao et al., 2013). As, the tumor inhibition and survivability efficacies of DOX/BSA-DEX-FA nanocomplex were evaluated on H22 tumor-bearing mice. The results were shown that tumors inhibition rates (n = 5 per every group) of the DOX which has been loaded nanocomplex are nearly same in comparison to free DOX at the same dose of 5 mg/kg. However, the groups treated with the nanocomplex increase their body weights considerably, while the free DOX group decreases the body weight extremely. Also, increasing the dose of nanocomplex led to enhance the tumor inhibition rate, whereas it was toxic to mice body for free DOX. These results suggest that the toxicity of targeted nanocomplex is so lower than the toxicity of free DOX and even DOX/BSA-DEX nanocomplex. Hao et al. (2013) were also investigated the nanocomplexes which have been produced by heating for 30 and 70 min. The results indicate that the nanocomplexes produced by heating for 30 min have better antitumor activity in comparison to other ones that it can be due to longer heat treatment and decomposition of the FA during the heat treatment.
In the other DEX-based DDS by Zhao et al. (2017), the resveratrol (RSV) as polyphenol was developed to cancer growth inhibition with antioxidant and antimutagen aspects. RSV can inhibit the cancers via activating the mitochondrial apoptotic pathway with induces apoptosis to inhibit cancerous cell proliferation and improves the sensitivity of drug resistant cancer cells (Mattarei et al., 2013). FA-DEX-RSV (mean particle size ∼140 nm) as polymeric micelle, has several great properties, including better stability compared to surfactant micelles, enhanced solubilizing power, longer circulating time which is owing to outer hydrophilic shell, small size, and targeting capability. The free RSV demonstrated around ∼20% of apoptotic fractions while FA-DEX-RSV indicated a threefold higher apoptosis. Moreover, drug release of FA-DEX-RSV nanomicelle was observed by ∼40 and ∼30% after 24 hr at acidic and normal pHs. Also, the cellular internalization of FA-DEX-RSV was more than eightfold in comparison to free RSV (Zhao et al., 2017). It was revealed that RSV-DF is associated with the higher expression of p53, caspase-3, and BCL2 associated X gene, apoptosis regulator (BAX) than the free RSV and higher level of BAX and caspase-3 was further demonstrated the involvement of mitochondria-dependent apoptosis in the anticancer efficacy of FA-DEX-RSV micelle (Zhao et al., 2017).
In recent decade, hyaluronic acid (HA) has also been widely evaluated for use in tumor-targeted DDS due to its ability in specifically bind to various cancer cells (Jing, Zhang, Zhou, Liu, & Zhang, 2013). HA, as anionic glycosaminoglycan, is a main component of the extracellular matrix that has major roles in cell proliferation and migration. Also, HA is a biodegradable, biocompatible, nontoxic, nonimmunogenic, and bioavailable polysaccharide. This polysaccharide is a ligand of CD44 hyaluronan receptors, which are overexpressed in a variety of tumor types including prostate and breast cancers (Choi et al., 2012; Jin et al., 2012). Furthermore, the self-assembled small size in aqueous conditions and enhance the enhanced permeability and retention (EPR) effects are other properties of this nanosized polymeric micelles. In a study by Qiu et al. (2014), the FA and poly(l-histidine) conjugated HA (FA-HA-PHis) micelle, as pH-responsive amphiphilic copolymer was developed for acid-triggered rapid release of DOX in cancer cells. The results were revealed that, FA-HA-PHis micelles (154.8 ± 1.6 nm in diameter) can selectively uptake via CD44 receptor-mediated endocytosis and rapidly disassemble in early endosomes which are owing to pH-induced protonation of PHis and hyaluronidase action. However, after endocytosis inhibition experiments, it was observed that FA-HA-PHis micelles were mainly internalized via clathrin-mediated endocytosis and DOX was delivered to lysosomes in this conditions (Qiu et al., 2014). On the other hand, the effect of the macropinocytosis pathway on the uptake of micelles was evaluated using colchicine (macropinocytosis pathway inhibitor) and the results were indicated reduction in the internalization (reduce to 3%) of targeted nanocomplex that imply macropinocytosis has main role in targeted-cell uptake. Self-assembled hydrophobized polysaccharide polymeric micelles are significantly exhibited high water-solubility for hydrophobic drug carrier, excellent solubilization capacity and stability, sustained drug release, prolonged circulation, and tumor localization aspects (Lee, Lee, & Park, 2008). Moreover, Liu et al. (2011) were synthesized dual targeting paclitaxel-loaded FA-HA-C18 micelles (FA-HA-PTX; 191.9 ± 8.7 nm in diameter) with drug encapsulation efficiency of ∼97.3% and drug release by ∼55.2% at 192 hr. Substantially, five endocytic pathways were known for surplus values of micelle internalization: FA-mediated endocytosis, clathrin-mediated endocytosis, caveolae-mediated endocytosis, macropinocytosis, and clathrin- and caveolae-independent endocytosis. These results were obtained by employing nanomolar dosages of several endocytic inhibitors such as hypertonic sucrose solution to dissociate of the clathrin, indometacin to inhibit the caveolae-mediated endocytosis, and chlorpromazine to dissociate the complex of clathrin and AP2 protein. However, FA- and clathrin-mediated endocytosis were considered as the most prominent mechanism for the micelle internalization (Liu et al., 2011).
2.2 Chemopolymers in drug delivery
2.2.1 Polyamidoamine NPs
Dendritic architecture is one of the most general topologies which has been observed in biological systems. Polyamidoamine (PAMAM) is one of the most studied dendrimers as the DDS (Figure 5). PAMAM NPs are nanosized (1–10 nm), three-dimensional, highly branched macromolecules which are consisting of three distinct components: a core, a hyperbranched mantle, and terminal functional groups (Narmani et al., 2017). These NPs are extremely soluble in water with many reactive end amine groups that make it as excellent nanocarrier for diagnostic and therapeutic goals (Narmani, Kamali, Amini, Salimi, & Panahi, 2018; Xie et al., 2014). In a research by Xu et al. (2016), FA and borneol (BO), well-known safe materials, which had been derived from traditional Chinese medicine, were applied for targeting delivery of DOX to glioma treatment. BO facilitates the BBB permeability and reduces the cytotoxic effects of PAMAM. On the other hand, the coupling BO on the surface of FA-PAMAM/DOX (167 nm in diameter) has several advantages for nanocomplex including increase its transportation from BBB (more than twofold), enhance its distribution agents in brain, prolong its circulation half-life time, improve DOX accumulation in brain tumor. Also, FA was improved the internalization efficiency until fourfold in cancer cell. Drug loading and entrapment efficiency of nanocomplexes were by 6.64% ± 0.09% and 64.58% ± 0.85%, and drug release of nanocomplex was by 28.2 and 48.8% over 24 hr at pH 7.4 and 5.5, respectively (Xu et al., 2016). For preclinical evaluation, the DOX plasma concentrations after intravenous injection of DOX, BO-PAMAM/DOX, and FA-BO-PAMAM/DOX were investigated via I.V. administration in rats (n = 5 per every group). As, DOX-loaded BO-PAMAM and FA-BO-PAMAM nanocomplexes were increased the area under the concentration-time curve (AUC0-inf) by 11.24- and 11.71-fold for BO-PAMAM/DOX and FA-BO-PAMAM/DOX, respectively. Furthermore, elimination half-life time (T1/2β) and mean residence time (MRT) of FA-BO-PAMAM were equal to 12.60 and 16.58 hr, while they were equal to 11.66 and 16.31 hr for BO-PAMAM, respectively. These results significantly support the extended residence time and control release profile of encapsulated drug in PAMAM as compared to free DOX in body (Xu et al., 2016). In another study, Narmani, Yavari, and Mohammadnejad (Narmani et al., 2017) were developed FA-PAMAM-PEG-5FU-99mTc in order to imaging and therapeutic goals. In the in vivo study, their synthetic nanocomplex was effectively targeted breast cancer cells (fourfold better than nontargeted complex) in tumor bearing mice (n = 3 per every group). Moreover, their targeted nanocomplex could be accumulated in tumor site after 4 hr and tumor accompanied with liver were absorbed more than 75% of nanocomplex.
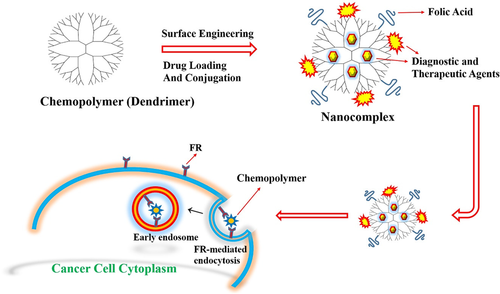
In the last decade, RNAs as anticancer agents have also been developed to target cell signaling intermediates to cancer cell inhibition. RNA-mediated therapeutic approaches can be selectively downregulated molecular targets in signaling pathways that are responsible for cell proliferation and significantly reduced multidrug resistance in comparison to drugs (Lo et al., 2010). Xu, Yeudall, & Yang, (2017) prepared FA-PAMAM NPs for active delivery of siRNA against vascular endothelial growth factor A (siVEGFA) in head and neck squamous cell carcinomas. The VEGFA is a main regulator of angiogenesis and promotes tumor development in tumor tissues, so that its knockdown has been shown to be effective in inhibiting tumor growth. FA-PAMAM-siVEGFA was exhibited FR-α mediated tumor uptake as excellent nanocomplex for local delivery of siRNAs and sustained retention properties. As it was considered, the very low systemic toxicity, sustained local drug release, high drug bioavailability, and excellent chemotherapeutic efficacy were advantages of this localized targeted delivery.
2.2.2 Polyethyleneimine NPs
Polyethyleneimines (PEIs) as cationic polymers have been applied as nucleic acids and drugs delivery carriers both in vitro and in vivo (Figure 5). Their surface positive charge, high drug loading, and good conjugation efficiency make them be able to combine with the therapeutic anticancer agents via electrostatic interactions (Benjaminsen, Mattebjerg, Henriksen, Moghimi, & Andresen, 2013). In a research by Wu, Zhang, Zhang, Sun, Wu, and Tang (2016), the PEG-b-(ɛCL-g-PEI)-b-ɛCL triblock copolymer (core–shell micelle) was applied for codelivery of P-glycoprotein (P-gp) siRNA and doxorubicin (DOX) to reverse MDR in breast cancer. The fabricated nanocomplex can effectively prevent renal clearance, RNase degradation and aggregation in circulation, minimize opsonization during circulation bloodstream, and protect micelles from mononuclear phagocytic system in circulation. After internalization, proton sponge effect of the branched PEI in the endosome help to endosomal elude of nanocomplex (Wu et al., 2016). The apoptosis levels of nanocomplex were measured by flow cytometric that they were obtained by 85.3, 41.3, and 15.7% for micelleplexes (PEG-DOX-b-(ɛCL-g-PEI)-b-ɛCL-siRNA), PEG-DOX-b-(ɛCL-g-PEI)-b-ɛCL, and free DOX, respectively. This high therapeutic efficiency of PEG-DOX-b-(ɛCL-g-PEI)-b-ɛCL-siRNA owes to overcome the drug efflux pump-mediated drug resistance. On the other hand, the histological analysis of tumor sections which has been stained with H&E was shown minimal residual cancer cells for micelleplexeses in comparison to free DOX. These results were indicated that micelleplexeses could considerably codeliver targeted nanocomplex to tumor tissues and subsequently produce more severe cancer cell apoptosis and tumor necrosis (Wu et al., 2016). Ultimately, the excellent internalization and effective tumor growth inhibition were observed in tumor bearing mice.
In other work by Shi et al. (2013), C60-PEI-FA nanocomplex was synthesized for targeting delivery of docetaxel (N-debenzoyl-N-tert-butoxy-carbonyl-10-deacetyl taxol, DTX) as a new class of hydrophobic taxane drugs with higher patient response rates and fewer side effects. DTX can inhibit the microtubule depolymerization to free tubulin in cancer cell. Fullerene (C60), as the third allotrope of carbon, is nanosize carbon material with unique photo-, electro-chemical, and physical properties which has excellent potency in delivery of hydrophobic therapeutic agents. This nanocomplex (140 ± 2.7 nm size in diameter) was indicated the prolonged blood circulation more than threefold tumor growth inhibition and apoptosis and 7.5-fold higher DTX uptake of tumor. Furthermore, the high tumor absorption through FR, which has been mediated internalization and EPR effect with low kidney clearance, was obtained in pharmacokinetic investigations (Shi et al., 2013). On the other hand, oligodeoxynucleotide delivery into cancer cells is a critical challenge in the cancer therapy. PEI NPs have been significantly developed to delivery of oligodeoxynucleotide vectors with high efficiency and low toxicity. In a study, Yang et al. (2015) were developed OA modified and FA decorated PEI NPs (FA-PEI-OA NPs) for delivery of LOR-2501 as antisense oligonucleotide against ribonucleotide reductase R1 subunit. OA could significantly improve the delivery efficacy of oligodeoxynucleotide. FA-PEI-OA-LOR-2501 nanocomplex was remarkably induced the downregulation of R1 mRNA and R1 protein. The higher cellular uptake was observed with PEI-OA/LOR-2501 at N/P ratio of 6. After inhibition of clathrin-mediated uptake in cancer cells, the clathrin-mediated uptake process was inhibited by 80.5% that demonstrated the clathrin-mediated internalization of nanocomplex (Yang et al., 2015). Also, the same results were obtained in cytosine deaminase/5-fluorocytosine (CD/5-FC) and TNF-related apoptosis-inducing ligand (TRAIL) genes delivery to glioma cells and rats via FA-PEG-PEI nanocomplex (N/P ratio of 15) that was reported by Liang et al. (2009). This research group was developed combined therapy of CD/5-FC with TRAIL genes against rat C6 glioma models. According to this study, average tumor size for the Phosphate-buffered saline (PBS)-control groups was equal to 172.52 ± 8.02 mm3, while 53.13 ± 3.72 mm3 noted for the combined therapy against glioma tumor (Liang et al., 2009). The cross-sensitization between TRAIL gene and 5-FC chemotherapeutic in this nanocomplex could induce apoptotic pathway through caspase activation and might be an effective therapeutic strategy for C6 gliomas.
3 LIPID-BASED NPS IN DRUG DELIVERY
3.1 Bilayer lipid NPs
Lipid-based NPs (e.g., solid lipid NPs and/or liposomes [LS]), as DDS, have been approved by US FDA for clinical use such as cancer biomarkers detection and its therapy. LS NPs with bilayer lipid architecture are spherical vesicles which have been formed by single or multiple lipid bilayers (Figure 6). These NPs provided better drug loading, excellent biodegradability, good stability characteristics, long circulation half-life, and easy surface functionalization (Huo et al., 2015; Nogueira, Gomes, Preto, & Cavaco-Paulo, 2015). Furthermore, capability to entrap both hydrophilic and hydrophobic drugs, nontoxic and lack of immune system activation are other advantages of LS NPs in DDSs (Huo et al., 2015). In a research by Sriraman, Salzano, Sarisozen, and Torchilin (2016), the PEGylated doxorubicin-loaded LSs targeted with FA, transferrin (Tf; FA-LS-DOX-Tf; with size 165 ± 33 nm in diameter and −21 ± 1.5 mV surface charge) were developed to cancer growth inhibition. The egg phosphatidylcholine, cholesterol, cholesteryl hemisuccinate, and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine were the main components of LS micelles. The dual-targeted LS micelles demonstrated sevenfold increase in cell accumulation compared to single ligand targeted ones. In clinical investigation, 6- to 8-week-old female athymic nude mice (n = 5) were inoculated on the right hind-flank with 4.5 × 106 HeLa cells in 100 μL of 50% v/v matrigel in serum-free Roswell park memorial institute culture medium (RPMI media). After 11th day, the mice were injected (I.V. administration) with a cumulative targeted nanocomplex and DOX dose of 4 mg/kg (100-μL injection volume for per day in five times). Tumor growth inhibition potency of targeted NPs was showed about 75, 79, and 34% for FA-targeted, dual-targeted micelles, and free DOX, respectively (Sriraman et al., 2016). The caspase-dependent apoptosis and topoisomerase II inhibition are DOX effects on cancer cells.
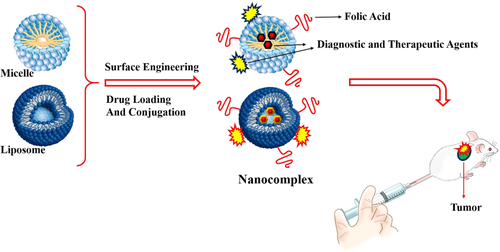
In other study by Lv et al. (2017), the capsaicin (CAP), as apoptosis inducer that could be instigate the intrinsic mitochondrial pathway and extrinsic death receptor pathways through caspase cascade in cancer cells, was loaded in 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000] (DSPE-PEG) FA-based lipid NP (with size 108.5 nm in diameter) as novel DDS. CAP with an increase in the p21 and decrease in cyclin E level, induce the cell cycle arrest, and cell death in G0/G1 phase of cell cycle. The higher cancer cell apoptosis (about 39%) with more than threefold internalization efficiency in comparison to nontargeted LS was exhibited in this research work (Zhang et al., 2008). Also, LS NPs which are owing to their high biocompatibility, favorable pharmacokinetic profile, high delivery efficiency, and ease of surface modification have been widely application in DDS for better clinical and translational development of targeted NPs in nanomedicine. However, some limitations of LS NPs such as insufficient drug loading, fast drug release, and instability in storage led to use hybrid NPs to overcome these disadvantages. Two methods including mix the polymeric NPs with liposomes to form the lipid-shell and polymer-core, and combines the nanoprecipitation method and the self-assembly technique are main approaches in synthesis of hybrid NPs (Zhang et al., 2008). Such hybrid NPs were synthesized by Liu, Li, Pan, Liu, and Feng (2010). They were developed PLGA-LS hybrid nanocomplex for targeted delivery of DTX as anticancer agent. Lipid layer was fabricated from three distinct functional components including (a) 1,2-dilauroylphosphatidylocholine, a phospholipid of an appropriate hydrophilic–lipophilic balance value as stabilizer of nanocomplex in the aqueous phase; (b) PEG modified 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE) to facilitate stealth NPs formulation to escape from recognition by the RES and subsequently increase the systemic circulation time of nanocomplex; and (iii) FA-functionalized 1,2-distearoyl-snglycero-3-phosphoethanolamine. This nanocomplex with size equal to 66.88 ± 0.67 nm in hydrodynamic diameter and surface charge of −20.74 ± 1.21 mV has been significantly shown better FR-β mediated cellular uptake respect to nontargeted nanocomplex (Liu et al., 2010).
3.2 Monolayer lipid NPs
In last decade, several lipid drug formulations have been approved for clinical use. However, some disadvantages of lipid-based NPs such as low drug loading, fast drug release, and low stability in storage led to use of monolayer lipid (LD) NPs (Figure 6) in combine with other NPs like polymers as core/shell micelles (Cho, Wang, Nie, Chen, & Shin, 2008). This mixed nanocomposite could be developed to combine the advantages and meanwhile overcome the disadvantages of both NPs. The PLGA, PLA, modified CS and PCL, PEG, and other bio-nanoparticles are very favorable NPs in combine with monolayer lipid NP due to their excellent biocompatibility and biodegradability, very good solubility both in water and organic solvents and high solubility and stability in aqueous solutions, desired pharmacokinetics, and prolonged blood circulation time (Cho et al., 2008; Jain, 2000). In a research by Zhao et al. (2012), the FA which has been functionalized PLGA and PEGylated octadecyl-quaternized lysine modified chitosan (FA-LD-PLGA-PEG) as core–shell micelles was synthesized for active delivery of PTX. PTX promotes the assembly and stabilization of microtubules and subsequently interferes with essential cellular functions including mitosis, cell transport, and cell motility. Subsequently, the particles size, surface charge, encapsulation efficiency, and loading efficiency of core–shell NPs were determined by 194 ± 7 nm, 22 ± 4 mV, 87% ± 2%, and 13% ± 1%, respectively. On the other hand, the core–shell NP was significantly shown controlled PTX release behavior (about two times sustained release respect to PLGA NPs and lipid NPs) with more than threefold tumor growth inhibition effects on tumor bearing animal models in comparison to pure PTX injection (Zhao et al., 2012). For the animal phase, 5- to 6-week-old severe combined immunodeficiency (SCID) female mice (18 ± 2 g, n = 9) were supplied in free access to sterilized FA-free food and inoculating human HeLa cells (1 × 107) were injected into the left flank of the mice for bearing cervix tumors. After treatment of mice via nontargeted and targeted nanocomplexes, by 22% mice were alive in PTX-LD-PLGA-PEG treated mice, whereas by 78% mice were alive in FA-PTX-LD-PLGA-PEG treated mice after 50 days treatment. Also, the biodistribution of nanocomplexes was investigated at 2 hr after tail intravenous injection of 10 mg/kg dose of nanocomplexes and results shown that drug concentration in tumor for FA-PTX-LD-PLGA-PEG was approximately equal to 3.70-, 1.85-, and 1.68-fold compared to that of PTX injection, PLGA NPs, and PTX-LD-PLGA-PEG, respectively (Zhao et al., 2012).
This kind of micelles comprise three distinct components including inner most polymeric core, lipid layer surrounding the polymeric core, and outer most PEGylated lipid covering which provides the good mechanical integrity, better penetration, excellent biocompatibility and in vivo stability, optimized drug entrapment, improve multiple drugs loading, and so forth. The carbodiimide click chemistry or thiol-maleimide approaches are main methods in surface modification of these micelles (Jain, 2000; Mandal, Mittal, Balabathula, Thoma, & Wood, 2016). In a study by Tahir et al. (2017), the methotrexate (MTX) which has been loaded lipid-polymer (PLGA/Lipoid S100) hybrid NPs was fabricated by employing a single-step modified nanoprecipitation method combined with self-assembly. The particles size and surface charges of different formulations varied between 308 and 176 nm, and equal to −21.2 ± 2.1 to −13.1 ± 3.1 mV, respectively, which increased with increasing polymer concentration. Furthermore, the in vitro drug release was also determined in range of 70.34–91.95% (Tahir et al., 2017). Also, the confocal fluorescence imaging was applied to determine the size of FITC-incorporated spherical NPs that was exhibited the particles with size less than 200 nm. In other work, Chen et al. (2013) were developed self-assembled FA-poly(ethylene glycol)-distearoyl-phosphatidyl-ethanolamine (PEG-DSPE) micelles in order to target delivery of mitoxantrone (MTN). The MTN and also dexamethasone or estrogen antagonist tamoxifen could be manipulated FR-α expression upregulation that induce the FR expression and significantly enhance the efficacy of targeted delivery via FR-α. These micelles with size about 40–60 nm were shown by 25.2% ± 2.2% encapsulation efficiency for MTN that were suitable for enhanced penetration into solid tumors. It was demonstrated that MTN which has been affected FR-α upregulation could be extremely enhanced the FA-mediated endocytosis efficiency of NPs (Chen et al., 2013). In another research by Hassanzadeh, Feng, Pettersson, and Hakkarainen (2015), the pluronic, F127 and reverse pluronic, and 10R5 were molecularly modulated for targeted codelivery of DOX and quercetin (QU) as hydrophilic agents. The QU which was covalently anchored to 10R5 DOX was loaded in to amphiphilic core–shell NPs (PEG-PPO-PEG, F127 and (PPO: poly propylene oxide) PPO-PEG-PPO, 10R5) with encapsulation capacity from ∼19 to ∼43%. Fundamentally, QU with interference on cell metabolism, Glutathione S-transferase (GST) activity, cytoskeleton, and invasive properties in tumor cells leads to improvement of DOX interactions with tumor cells. Furthermore, stabilize the micelles and decrease the critical micelle concentration (CMC) by adding a hydrophobic molecule to pluronic 10R5. The pluronics as bioavailable and biocompatible molecules could inhibit P-gp and sensitize MDR cells as a result of Adenosine triphosphate (ATP) depletion which subsequently decrease drug resistance in the cancerous cells. Moreover, pluronics, due to their high CMC, conjugated with PEG in order to enhance their stability and decrease dilution in the blood after intravenous administration.
4 METAL- AND NONMETAL-BASED NPS IN DRUG DELIVERY
4.1 Iron oxide NPs
Magnetic iron oxides (Fe3O4) NPs (MNP) are widely applied in biological and biomedical fields due to their specific magnetic and tunable physicochemical aspects (Ladj et al., 2013). These properties make practical applications for MNPs in diagnostic and therapeutic fields including cell labeling and targeting, active drug delivery, magnetic resonance imaging (MRI), hyperthermia, magnetofection, and so forth (Figure 7). Furthermore, the good colloidal stability, biocompatibility, and long blood circulation time are also other important properties of MNPs (McBain, Yiu, & Dobson, 2008; Varshosaz et al., 2013). On the other hand, the modification of hydrophilic and noncytotoxic polymers such as dextran, dendrimers, CS, PEG, and PEI on the surface of MNPs could effectively improve their colloidal stability and biocompatibility of MNPs (Yang et al., 2014). In a study by Li et al. (2013), the PEI-mediated approach was developed to synthesize FA-targeted MNPs for in vivo MRI, as one of the powerful and noninvasive imaging techniques because of its high spatial resolution and tomographic capabilities. As a matter of fact, PEI-coated MNPs were prepared by a one-pot hydrothermal route and these aminated MNPs were subsequently decorated with FITC and FA-conjugated PEG (FA-PEG-FITC-PEI-MNPs; with size equal to 288.7 ± 6.90 nm in hydrodynamic diameter and equal to 16.3 ± 0.17 mV in surface charge). They were indicated that the PEI-mediated approach along with the PEGylation enables the generation of water-dispersible and stable multifunctional MNPs. The in vitro investigations were also confirmed the cytocompatibility, hemocompatibility, and cancer cell targeting of nanocomplex. On the other hand, the spin–spin relaxation times (T2) relaxivity measurements were indicated that FA-PEG-FITC-PEI-MNPs have a higher r2 value than the MNPs which have been coated with polymer multilayers which is essential for them to be used as good T2 negative contrast agent for sensitive MRI applications. For in vivo experiments, the male 4- to 6-week-old BALB/c nude mice (15–20 g, n = 5) were subcutaneously injected with 2 × 106 cells/mouse in the left back and when the tumor nodules reached a volume of 0.8–1.4 cm3, the Fe3O4-PEI-Ac-FI-PEG-FA NPs were administrated into the nude mice. Subsequently, the T2-weighted MR images of the xenografted (KERATIN-forming tumor cell line HeLa) KB tumor obtained before injection, and 30 min, 1 hr, 2 hr, 4 hr, and 24 hr post-injection and tumor MR signal of mice which has been treated with targeted nanocomplex does not decrease with time post-injection (Li et al., 2013). On the other hand, the Fe biodistribution analysis was indicated that PEG-modified nanocomplex can help to the Fe particles to escape from the reticuloendothelial system and deliver to the tumor tissue.
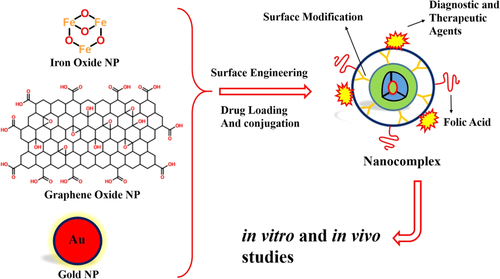
In a research by Huang, Mao, Zhang, and Zhao (2017), MNPs co-coated with PEG and PEI polymers were synthesized by an improved polyol method in order to target delivery of DOX and imaging (theranostics) with mean hydrodynamic diameters of 67 nm. Excellent FA receptor-mediated endocytosis was implicated by means of confocal laser scanning microscope. Also, drug-loading capacity and drug-loading efficiency of theranostic nanocomplex (FA-PEG-PEI-DOX-MNPs) were by 29.3 and 78.5%, respectively, that was indicated total DOX released about 90% in PBS at pH 5.0 in 48 hr, which was significantly higher than that at pH 7.4. Moreover, the external magnetic field was enhanced about 6.9-fold of the accumulation of the injected dose in tumor of the mice which has been treated with FA-PEG-PEI-DOX-MNPs. Eventually, the nanocomplex was induced a higher inhibition in tumor growth as compared to those treated with free DOX and MNP. On the other hand, the T2 relaxivity of nanocomplexes were measured by a 3.0T MRI system in order to evaluate the T2-weighted MR imaging performance and results were shown that nanocomplex was negative T2 contrast agent (Huang et al., 2017). Also, the in vivo investigation on nude mice with MCF-7 xenograft (n = 5 per group) was demonstrated that tumor sizes of the mice which have been treated with FA-PEG-PEI-DOX-MNPs in the presence of external magnetic field are remarkable reduction tendencies. Substantially, the polymers owing to good biocompatibility, (laboratory-bred strain of the house mouse) drug encapsulation, and controlled release can be considered as excellent candidate for MNPs surface modifications (Huang et al., 2017). The Saikia, Das, Ramteke, and Maji (2017) were developed the genipin cross-linked aminated starch/ZnO nano-composite for surface modification of MNPs to target delivery of Cur (FA-S/ZnO-Cur-MNPs). At the lower pH, the positive charges generated on aminated starch caused by the protonation of the amine groups which have been facilitated the repulsion between the polymer chains and thus increased the drug release of nanocomplexes. They were found that NPs with 5% ZnO (with size 31.2 nm) indicated highest cellular uptake among the ZnO incorporated NPs. Furthermore, the reactive oxygen species (ROS) estimation study was exhibited that with the increase in ZnO concentration, the generation of ROS increased in cancer cells. These results were significantly shown the highest cellular uptake of nanocomplex (Saikia et al., 2017). In another study, Sadhasivam, Savitha, Wu, Lin, and Stobinski (2015) were successfully prepared carbon encapsulated MNPs (CMNP: carbon encapsulated-magnetic iron oxides nanoparticles) (FA-PEG-CMNP) by carbon arc method for in vitro magnetic hyperthermia. These dual-targeted MNPs (with size 70–90 nm) have generated quick heating (43–45°C) upon exposure to an alternating magnetic field. In hyperthermia, as active cancer therapy, the certain body tissues are exposed to the temperature range of 41–46°C to damage and kill cancer cells. The magnetic hyperthermia efficiency was evaluated by Lactic dehydrogenase (LDH) cytotoxic assay and the MNPs were nontoxic to normal human cells under the alternating magnetic field (Sadhasivam et al., 2015).
4.2 Graphene oxide NPs
Graphene oxide (GO), as a derivate of graphene, has many surface functional groups such as carboxylic acid (on the sheet edges) and hydroxyl groups (on the basal plane) (Figure 7; Liu, Robinson, Sun, & Dai, 2008). The special structural characteristics, high physiological stability, excellent biocompatibility, and nontoxicity make it as high potency NPs for various biomedical applications including drug/gene delivery, biosensing, photothermal therapy, and bioimaging (Ahadian et al., 2015; Liu et al., 2008). Moreover, the GO NPs provide a large specific surface area for the immobilization of anticancer agents (Hong, Compton, An, Eryazici, & Nguyen, 2011). In a research by Ma et al. (2017), FA-grafted bovine serum albumins (FA-BSA) were immobilized on GO NPs for delivery of DOX as stabilizer and targeting agents. The FA-BSA was decorated on the surface of GO NPs by the physical adsorption. Also, DOX was associated with GO NPs through π–π and hydrogen-bond interactions, resulting in high drug loading. The FA-BSA-DOX-GO nanocomplex was exhibited pH responsive (high drug loading in acidic condition) and sustained drug release. Furthermore, high drug loading was obtained in 3:1 mass ratio of DOX to FA-BSA/GO. The FA-BSA/GO, as biocompatible and biodegradable nanocomplex, was shown less than 5% of hemolysis ratio (Ma et al., 2017).
In another work, Wang et al. (2013) were employed dual-targeted DDS using FA and MNPs bifunctionalized GO. The magnetic field as external targeting strategy improves drug delivery efficiency affecting the MNPs. They used CS as a bridge to combine FA with functional GO, enhance the stability and biocompatibility of nanocomplex and improve the encapsulation efficiency and control release of drug molecules. Moreover, pH-dependent drug release and drug-loading capacity (0.98 mg/mg) were observed which are owing to the different degree of hydrogen bonding interaction between DOX and the FA-DOX-MNP-GO nanocomplex. Also, it was revealed that DOX release may be related to the degradation of CS. On the other hand, CS degradation is faster in acidic pH of 5.3 (Wang et al., 2013). In another research by Qin et al. (2013), the FA and polyvinylpyrrolidone (PVP) functionalized GO NPs (FA-PVP-GO NPs) were developed for targeted near-infrared (NIR) photothermal therapy and DOX delivery (with the loading ratio more than 100%). PVP was employed as a stabilizing agent and dispersant in the synthesis of metal nanostructures. Furthermore, PVP as a biocompatible stabilizer could improve the GO NPs stability in physiological environment. On the other hand, NP-based NIR photothermal therapy provides a promising treatment strategy for efficient tumor ablation with minor injury to the surrounding tissue. GO NPs, due to their strong absorbance in the NIR region, have been employed to photothermal therapy. Moreover, GO NPs indicated concentration-dependent and time-dependent temperature increase in response to NIR irradiation and its extraordinary photothermal energy conversion efficiency was remarkably demonstrated. The FA-PVP-GO generated heat more efficiently (ΔT ≈ 50°C at 10 μg/mL, 5 min) in the same conditions than PVP-GO (ΔT ≈ 40°C at 10 μg/mL, 5 min; Qin et al., 2013). Both FR-mediated internalization and mitochondrial accumulation, and cell surface attachment of these NPs could enhance the sensitive of cells to NIR-mediated photothermal damage. These effects lead to cytochrome c release and subsequently cell apoptosis.
4.3 Gold NPs
In the last decades, gold NPs (GNPs), due to their unique fluorescence, low cost, and so forth have been developed extensively to establish powerful optical methods for sensing and biomedical applications (Figure 7; Narmani, Kamali, Amini, et al., 2018; Peng et al., 2012). Owing to higher atomic number and electron density, GNP fundamentally has a higher X-ray absorption coefficient that makes it as suitable candidate for diagnostic and therapeutic applications. Also, GNPs are easy to modify the help to eliminate their low cytotoxicity and avoid removal activity of RES after proper surface functionalization (Cheng et al., 2013). On the other hand, tumor cell targeted GNPs can effectively induce the DNA damage, cytokinesis arrest, and apoptosis (Cheng et al., 2013; Lu et al., 2010). In a study by Wang, Zheng, Peng, Shen, Shi, and Zhang (2013), the FA-modified polyamidoamine-entrapped GNPs (FA-PAMAM-GNPs; with size 3.1 nm in diameter) as nanoprobes, were employed for in vitro and in vivo targeted computed tomography (CT) imaging. They were used amine-terminated generation 5 poly(amidoamine) dendrimers as platforms of covalently linked FA, followed by an acetylation reaction to neutralize the remaining dendrimer surface amines. Micro-CT images were indicated that cancer cells can be detected under X-ray after intravenous and intraperitoneal administration of FA-PAMAM-GNPs. The nanocomplex was considerably penetrated in tumor vasculature through its leaky endothelium and accumulated in solid tumors via the EPR. The silver enhancer staining (using silver enhancement kit) and the FR immunohistochemistry staining on xenografts SPC-A1 tumor cells were shown more than 10-fold of cellular uptake of FA-PAMAM-GNPs nanocomplex in tumor cells and also the higher magnification images of Transmission electron microscopy (TEM) were dominantly demonstrated the nanocomplex accumulation in the lysosomes. For the FR immunohistochemistry staining, the SPC-A1 cells which were growing on polylysine-coated cover slips were prepared and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer. Then tumor tissue sections were boiled in 0.01 M citrate buffer and they were cooled for FR immunohistochemistry. Subsequently, sections were blocked with 1% normal goat serum (blocking buffer) and then incubated with primary polyclonal anti-FR antibody that fallowed with secondary biotinylated antibodies incubation. After washing, sections were incubated with secondary biotinylated antibodies and then images were captured using light microscope (Wang, Zheng, Peng, Shen, Shi, & Zhang 2013). In addition, flow cytometry analysis was shown cell cycle and cell apoptosis followed the order of G0-G1˃G2-M˃S˃G2/G1. On the other hand, the biodistribution investigation was exhibited excellent tumor and spleen uptake and it was indicated that nanocomplex can be cleared mainly through the renal/urinary route and RES (Wang, Zheng, Peng, Shen, Shi, & Zhang 2013). Due to deep tissue penetration, better spatial and density resolution than other imaging modalities, CT has been considered as most common imaging techniques in cancer diagnostic. On the other hand, the intratumoral, intravenous, and intraperitoneal injection routes were evaluated on animals to determine the best administration route for CT imaging. Results were indicated that intravenous injection is the most promising way to introduce its usage in clinical trials.
In another work, Zhou et al. (2016) were developed a cost-effective contrast agent for tumor CT imaging. They were fabricated PEGylated polyethylenimine-entrapped gold NPs which have been functionalized with FA and FITC for active tumor CT imaging. PEGylation of PEI could enhance the entrapment of GNPs within its interior (FA-FITC-PEG-PEI-GNPs, with a mean diameter of 202.4 nm). Subsequently, the X-ray attenuation property of FA-FITC-PEG-PEI-GNPs was indicated that all three materials display which have been increased CT contrast enhancement and nanocomplex have a great potential to be used for CT imaging applications. Also, the flow cytometry and confocal microscopic imaging data exhibited that FA can effectively increase the cellular uptake more than threefold in cancer cells of tumor (Zhou et al., 2016). Furthermore, the tumor biodistribution investigation was revealed that the Au uptake in all the major organs started to decrease after 72 hr post-injection, and Au uptake is just equal to 39.4, 37.5, and 15.6% in the liver, spleen, and lung, respectively. These evaluations were demonstrated that the NPs can be effectively metabolized and cleared out the body without exhibit any side effect in body normal organs (Zhou et al., 2016). On the other hand, in a research by Li, Cheng, Liu, and Chen (2016), the FA-BSA functionalized noble gold nanoclusters (FA-BSA-GNP) were employed to imaging of FR which has been overexpressed tumor cells. In this work, Li et al. were reported a simple, cost-effective, turn on, and red fluorescent probe for FR overexpressed tumor cells based on the recovery of fluorescence intensity of FA-BSA-GNP nanocomplex. In this assay, first primary fluorescence intensity of FA-BSA-GNP was quenched via FA-mediated the environment change of FA-BSA-GNP to produce negligible fluorescence background. Subsequently, primary fluorescence intensity of FA-BSA-GNP turned on by FA desorbing from FA-BSA-GNP which is owing to specific affinity of FA and FR (Li et al., 2016).
5 UPCONVERSION NPS IN DRUG DELIVERY
Upconverting NPs (UCNPs) are inorganic crystalline nanomaterials that are able to convert low-energy NIR (photons with 980 or 808 nm wavelength) excitation light into visible and ultraviolet emission light (Figure 8; Zhou, Liu & Li 2012; Shen et al., 2013; Auzel, 2004). UCNPs are promising for applications such as biological imaging and drug carriers in biology and medicine. Fundamentally, this photophysical phenomenon is based on an anti-Stokes process and referred to as photon upconversion where two or more photons are absorbed by a material and a single photon of shorter wavelength (anti-Stokes shift) is subsequently emitted (Rinkel, Nordman, Raj, & Haase, 2014; Narmani, Farhood, Haghi-Aminjan, et al., 2018; Han, Deng, Xie, & Liu, 2014; Boyer, Carling, Gates, & Branda, 2010). The excited state absorption, energy transfer upconversion, and photon avalanche are various mechanisms of upconversion emission (Zhou, Liu & Li 2012; Wang et al. 2009). Furthermore, the uniform size and morphology, narrow emission peaks, large anti-Stokes shifts, excellent photostability, nonphotoblinking, long lifetimes, high crystallinity, good dispersibility in aqueous solution, and presence of suitable functional surface groups are specific properties of UCNPs (Rinkel et al., 2014; Wang et al., 2006). In a study by Cao et al. (2011), the FA which has been functionalized rare-earth up conversion nano-phosphors, as excellent luminescent labels, were developed for fluorescence bioimaging. They were synthesized high-quality water-soluble UCNPs using a hydrothermal reaction assisted by binary cooperative ligands by one-step synthetic strategy. Their research team used 6-aminohexanoic acid and oleate (as the chelating agent and surfactant) to control nuclear generation and crystal growth of small NPs. Moreover, these two compounds make UCNPs water-soluble to conjugate with FA. Eventually, the rare-earth activator ions including Er3+, Tm3+, and Ho3+ were doped to nanomaterial to exhibit upconversion luminescence (UCL) in imaging process that Tm3+ was sued in Cao et al. (Cao et al., 2011) research. The clear UCL signal at 800 nm was significantly detected at lymphatic and the removed axillary lymph nodes were also indicated strong UCL in the ex vivo image due to specific FR targeting. The in vivo UCL imaging was performed for lymphatic mapping on mouse models. For this evaluation, two external 0–5 W adjustable CW (infrared laser) and 980 nm lasers were used as the excitation sources. Then, the images of luminescent signals were analyzed and UCL signals were subsequently collected at 800–12 nm. For achieve to this evaluation, animals were placed in the prostrate position while under pentobarbital anesthesia. Then, 20 mL (1 mg/mL) of nanocomplex injected into the paw of the nude mice for 20 min and eventually the UCL lymphatic imaging was performed (Cao et al., 2011). Furthermore, lymphadenectomy was exhibited strong UCL in the ex vivo image of removed axillary lymph nodes. These results were indicated that detection of lymph nodes could help to clinical lymph nodal excision surgery of tumor resection.
In a research, Zeng et al. (2015) were fabricated FA targeted, photosensitizer (PS)-loaded Fe3O4-NaYF4:Yb/Er nanocomplex for in vivo T2-weighted MRI and visualized photodynamic therapy (PDT) of breast cancer. This nanocomplex (with size 175 nm in diameter, 12.5 mV in surface charge) was indicated NIR-triggered PDT performance which is owing to the production of singlet oxygen species. The UCL and singlet oxygen species are important factors to investigate NIR-triggered PDT of Er-doped UCL nanomaterials. On the other hand, the transverse MR relaxivity of 63.79 mM/s (r2 value) and in vivo MR imaging were exhibited an excellent T2-weighted MRI. Also, the in vivo PDT performance of nanocomplex was assessed on MCF-7 tumor-bearing nude mice (n = 5), and change of relative tumor volume was evaluated between 15-day treatments. As relative volume of MCF-7 tumors was equal to 3.16, 2.46, and 2.89 for mice that treated with PBS, PBS + NIR, and nontargeted nanocomplex, respectively, however it was equal to 0.16 in group of FA-NPs + NIR. Moreover, H&E staining for tissue sections of mice normal organs was not indicated fibrosis in the heart and lung samples and inflammatory reaction in the liver section and also normal glomerular and tubular structures were exhibited for kidney (Zeng et al., 2015). Furthermore, the Fe3O4-NaYF4:Yb/Er NPs were remarkably accumulated in liver and tumor due to the extra uptake of Kupffer cells in liver and FA-mediated internalization and EPR effect of nanocomplex in tumor (Zeng et al., 2015). In another study by Ma et al. (2012), FA-conjugated silica which has been modified LaF3:Yb, Tm, Ho, Er UCNPs (FA-SiO2-UCNPs, with an average size of 6.56 ± 1.16 nm in diameter and −40.39 ± 3.22 mV in surface charge) with high La content were prepared for simultaneously targeting dual-modality imaging of UCL and X-ray CT. In this study, UCL properties of FA-SiO2-UCNPs were codoped with Yb3+ and Er3+/Tm3+/Ho3+. Substantially, nanocomplexes were synthesized by a novel OA/ionic liquid two-phase system that subsequently were functionalized via FA. The FA-SiO2-UCNPs were indicated good stability, good biocompatibility, highly selective targeting, great water solubility, excellent X-ray attenuation, and UCL emission under excitation at 980 nm (Ma et al., 2012). On the other hand, Wang, Cheng, and Liu (2011) were functionalized Yb3+ and Er3+ doped NaYF4 UCNPs with a PEG-grafted amphiphilic polymer and FA (FA-PEG-NaYF4 UCNPs) for intracellular active drug delivery and UCL imaging. UCNPs are loaded with a DOX as chemotherapy molecule, by simple physical adsorption via a supramolecular chemistry approach. The releasing of DOX from UCNPs (with drug-loading efficiency of 8% w/w) was controlled by varying pH and exhibited an increased drug dissociation rate (more than twofold) in acidic environment (Wang et al., 2011).
6 CONCLUSION
In this review, we discussed various FA-functionalized NPs which have been widely utilized for the development of active cancer diagnosis and therapy. NPs-based active delivery systems hold excellent potential to overcome some disadvantages of the conventional therapeutic and diagnostic approaches. These systems have specific properties due to their various advantages, including their nanosize range, great stability, biocompatibility, bioavailability, biodegradability, nontoxicity, good loading capacity, potential for active tumor accumulation, overcome to problems of drug resistance in cancer cell, and excellent pharmacokinetic parameters. Furthermore, surface functionalized NPs can improve the specific drug delivery into cancer cells and FA targeting shows considerable promise for development of active cancer diagnosis and therapy. Several studies against various tumors FR have tested for specific drug delivery into tumor tissues. However, challenges still remain in clinical trials for developing FR-targeted NPs against human cancer cells. Moreover, an excellent understanding of FA-functionalized NPs in clinical translations is needed to improve their performances for therapeutic and diagnostic practical applications. Actually, evaluation of interaction between FA-functionalized NPs and complex biomolecules in human body serum, determination of pharmacokinetic and pharmacodynamic facts about FA-functionalized NPs, accurate evaluation of FA-functionalized NPs biodistribution in human body, and probable cytotoxic behavior and subcellular fate of FA-functionalized NPs are the main challenges of FA-decorated NPs which need to more assessments in preclinical and clinical trials. On the other hand, there is little understanding regarding the practical applications of FA-functionalized NPs in human tumor models; however, we believe that these NPs are most promising nanoplatforms for the future of targeted DDSs. Therefore, it is necessary to design the comprehensive studies regarding clinical trials in various cancer diseases to obtain more information about the potency of FA-functionalized NPs in human cancer diagnosis and therapy.