Reviewing on AI-Designed Antibiotic Targeting Drug-Resistant Superbugs by Emphasizing Mechanisms of Action
ABSTRACT
The emergence of drug-resistant bacteria, often referred to as “superbugs,” poses a profound and escalating challenge to global health systems, surpassing the capabilities of traditional antibiotic discovery methods. As resistance mechanisms evolve rapidly, the need for innovative solutions has never been more critical. This review delves into the transformative role of AI-driven methodologies in antibiotic development, particularly in targeting drug-resistant bacterial strains (DRSBs), with an emphasis on understanding their mechanisms of action. AI algorithms have revolutionized the antibiotic discovery process by efficiently collecting, analyzing, and modeling complex datasets to predict both the effectiveness of potential antibiotics and the mechanisms of bacterial resistance. These computational advancements enable researchers to identify promising antibiotic candidates with unique mechanisms that effectively bypass conventional resistance pathways. By specifically targeting critical bacterial processes or disrupting essential cellular components, these AI-designed antibiotics offer robust solutions for combating even the most resilient bacterial strains. The application of AI in antibiotic design represents a paradigm shift, enabling the rapid and precise identification of novel compounds with tailored mechanisms of action. This approach not only accelerates the drug development timeline but also enhances the precision of targeting superbugs, significantly improving therapeutic outcomes. Furthermore, understanding the underlying mechanisms of these AI-designed antibiotics is crucial for optimizing their clinical efficacy and devising proactive strategies to prevent the emergence of further resistance. AI-driven antibiotic discovery is poised to play a pivotal role in the global fight against antimicrobial resistance. By leveraging the power of artificial intelligence, researchers are opening new frontiers in the development of effective treatments, ensuring a proactive and sustainable response to the growing threat of drug-resistant bacteria.
1 Introduction
Drug-Resistant Superbugs (DRSBs) are a significant global health concern (Algammal et al. 2023). These superbugs, such as methicillin-resistant Staphylococcus aureus and multidrug-resistant Escherichia coli, pose a threat to critically ill patients due to their resistance to multiple antibiotics (Kalita et al. 2021). These superbugs, resistant to multiple antimicrobials, including antibiotics, are becoming increasingly difficult to treat, leading to higher mortality rates and healthcare costs. Diseases like tuberculosis, caused by drug-resistant microbes, are particularly challenging to manage, especially in patients with co-infections like HIV (Elfirdaous Fari et al. 2023).
Novel strategies to combat multidrug resistance (MDR) include Nontraditional approaches like quorum sensing-targeted therapy, nanoparticle-based therapy, anti-virulence therapy, bacteriophage therapy, and antimicrobial peptides (Parmanik et al. 2022). Nanotechnological approaches, such as nano-drugs with unique properties, are proving effective against MDR. Exotoxin-targeted therapies and microbiome therapy are advanced Nontraditional approaches to treat infections caused by pathogens like Staphylococcus aureus and Clostridium difficile (Pramanik et al. 2023).
Nanotechnological approaches, such as the use of reduced graphene oxide (r-GO) and WO3 nanowire-based photothermal-photocatalytic heterostructures, have shown promising results in killing superbugs like Salmonella DT104, carbapenem-resistant E. coli, and methicillin-resistant S. aureus (Parmanik et al. 2022). The search for effective solutions against DRSBs emphasizes the importance of rational antibiotic use and hygiene practices to prevent further resistance development (Yadav et al. 2023). Artificial intelligence (AI) strategies are increasingly being explored to combat multidrug resistance (MDR) globally. Nontraditional approaches like quorum sensing (QS)-targeted therapy, nanoparticle-based therapy, and antimicrobial peptides are gaining attention in addressing MDR (Khan and Torchilin 2022). Furthermore, the use of AI in nanomedicine-based strategies, such as nanoparticle-/liposome-based drug delivery and gene regulation through siRNA, is enhancing therapeutic responses and overcoming resistance in cancer treatment (Lin et al. 2023).
Antimicrobial peptides (AMPs) designed using computational techniques target specific bacterial proteins like BamA, disrupting outer membrane protein folding and exhibiting potent antibacterial effects against drug-resistant Gram-negative bacteria (Mishra et al. 2024). Figure 1.
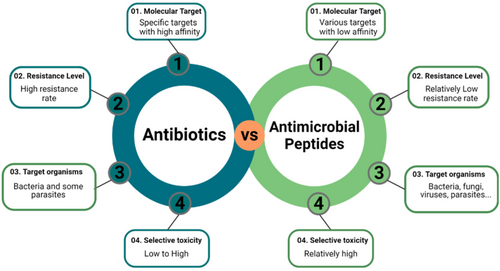
Artificial intelligence [AI] plays a crucial role in accelerating the discovery of novel antibiotics, including AMPs, by predicting antimicrobial activity, drug-likeness traits, and resistance patterns (Figure 2) (Mukherjee et al. 2022).
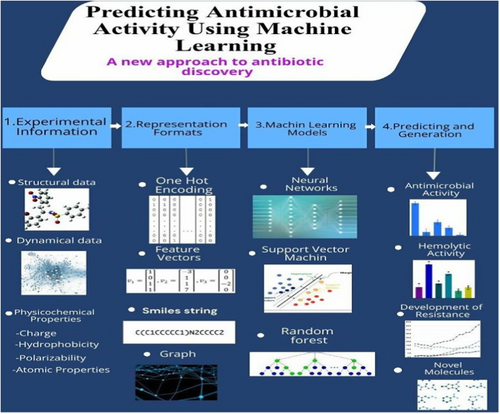
AI-driven approaches have successfully generated AMP candidates effective against both general and antibiotic-resistant bacterial strains, showcasing a rapid and efficient method for peptide design and synthesis (Lau et al. 2021). Additionally, AI platforms like IDentif. AI have been instrumental in optimizing combination therapy regimens against highly resistant bacteria like Mycobacterium abscessus, outperforming standard of care regimens and offering new treatment options (Wu et al. 2024). The integration of AI and machine learning algorithms in antimicrobial research holds promise for combating the global health crisis of antimicrobial resistance by accelerating drug development and identification of effective treatments. This study aims to investigate the potential of Artificial Intelligence (AI) for designing novel antibiotics effective against Drug-Resistant Superbugs (DRSBs). The primary focus will be on elucidating the mechanisms of action employed by DRSBs and how AI can be leveraged to target these mechanisms for antibiotic development. Figure 3.
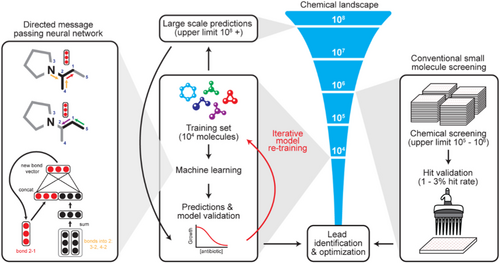
2 Methods
The methods employed for investigating AI-designed antibiotics targeting drug-resistant superbugs with an emphasis on mechanisms of action included a systematic literature review, conducted to identify relevant articles from databases such as PubMed, Scopus, and Web of Science. Keywords like “AI-designed antibiotics,” “drug-resistant bacteria,” and “mechanisms of action” were used to refine the search results. Data extraction and analysis were carried out on selected studies, focusing on the development and mechanism of action of AI-designed antibiotics. Detailed information regarding the design principles, computational methods, and experimental validation of these antibiotics was extracted and synthesized.
Mechanistic insights were gained by examining the underlying mechanisms of action that contribute to the efficacy of AI-designed antibiotics against drug-resistant superbugs. Special attention was paid to understanding how AI algorithms identify and exploit vulnerabilities in bacterial targets, thereby enhancing antibiotic efficacy. Case studies illustrating the application of AI-driven approaches in antibiotic development were reviewed, with a particular focus on their mechanism of action against drug-resistant pathogens. These case studies included examples of AI-designed antibiotics that showed promising results in preclinical and clinical studies.
A critical evaluation of the strengths and limitations of AI-driven antibiotic development was conducted, addressing considerations such as computational accuracy, scalability, and regulatory challenges. The findings from the literature review, data extraction, and mechanistic insights were synthesized to provide a comprehensive understanding of AI-designed antibiotics targeting drug-resistant superbugs. Ethical considerations surrounding the use of AI in antibiotic development were also discussed to ensure responsible research conduct in this domain.
-
Mitigating Algorithmic Bias: We prioritized studies utilizing diverse and high-quality datasets that represented a wide range of bacterial species and strains. Research employing transparent model training, cross-validation, and external validation techniques was emphasized to enhance generalizability. Mechanism-focused and explainable AI approaches were highlighted to ensure interpretability and fairness in feature selection and predictions.
-
Enhancing Scalability in Resource-Limited Settings: We evaluated studies leveraging lightweight AI models, transfer learning, and open-source platforms to reduce computational resource dependency. Cloud-based and decentralized solutions were reviewed for their potential to extend access to advanced AI tools. Additionally, the integration of localized bacterial data and cost-effective data collection methods, such as in silico simulations, was emphasized to maximize impact in resource-constrained environments.
These strategies ensured a balanced, scalable, and globally relevant analysis of AI-driven approaches for antibiotic discovery.
3 Results
In a significant development for public health, researchers at Harvard University have created a new antibiotic that shows promise in overcoming the growing threat of drug-resistant bacteria. This new compound, named cresomycin, is particularly effective against strains like Staphylococcus aureus and Pseudomonas aeruginosa, which are responsible for millions of deaths worldwide. The effectiveness of cresomycin stems from its unique design. While traditional antibiotics target bacterial ribosomes, some bacteria have developed defenses to render these drugs ineffective. Cresomycin, however, is specifically engineered to bypass these defenses. It binds more tightly to ribosomes due to its pre-organized structure, preventing bacteria from using their resistance mechanisms (Pérez-Moreno et al. 2024).
Researchers also discovered clovibactin by cultivating previously unstudied bacteria from soil. This discovery highlights the potential of “bacterial dark matter,” which represents a vast reservoir of untapped resources for developing new antibiotics. Unlike existing antibiotics, clovibactin works through a unique mechanism that targets multiple essential molecules in bacterial cell walls. This multi-pronged approach makes it difficult for bacteria to develop resistance. Studies have shown effectiveness against a range of gram-positive pathogens, including some particularly troublesome superbugs. The drug also exhibits low toxicity to human cells, suggesting minimal side effects (Pérez-Moreno et al. 2024).
Researchers at the University of Liverpool have also developed teixobactin, a naturally occurring antibiotic with potent activity. Notably, the new versions proved effective against bacteria resistant to current antibiotics, including biofilms, which are particularly difficult to treat. This research offers significant hope for the development of new treatments for serious chronic infections (Parmar et al. 2023). Da Cunha et al. utilized machine learning, spectroscopy, and Fourier-transform infrared spectroscopy to investigate antibiotic mechanisms and potency. They detected metabolic fingerprints to assess growth inhibition and predict mechanisms of action within antibiotic classes (Ribeiro da Cunha et al. 2021).
The integration of artificial intelligence (AI) into strategies targeting bacterial biofilms and resistance mechanisms is paving the way for innovative solutions to antimicrobial resistance. By enhancing the design of antimicrobial agents and advancing the detection and understanding of biofilms, AI offers promising avenues for improving treatment effectiveness.
3.1 AI in Designing Antimicrobial Peptides
AI-driven frameworks, such as explainable artificial intelligence (XAI), play a pivotal role in the rational design of antimicrobial peptides (AMPs) that combat biofilms. For example, research by Pikalyova et al. (2024) demonstrated a remarkable 100% success rate against methicillin-resistant Staphylococcus aureus (MRSA) using peptides generated through AI. Machine learning algorithms further optimize peptide properties, boosting their effectiveness while simultaneously reducing production costs (Pikalyova et al. 2024).
3.2 Advances in Biofilm Detection and Characterization
AI-powered methods, including machine learning and advanced image processing, significantly enhance the identification of biofilm-forming pathogens across diverse surfaces. These advancements, highlighted by Mishra et al. (2024), are instrumental in enabling prompt intervention. By providing faster diagnostics and facilitating tailored treatment approaches, AI addresses key challenges posed by biofilms in clinical environments (Mishra et al. 2024).
3.3 Emerging Therapeutic Innovations
Small-scale robotics offer a groundbreaking approach to treating biofilm infections by combining mechanochemical disruption with localized drug delivery systems. Additionally, AI supports the refinement of antimicrobial photo-sonodynamic therapy by predicting resistance patterns and customizing treatment regimens, as noted by Pourhajibagher et al (Pourhajibagher et al. 2024). These developments underscore the potential of AI to revolutionize treatment approaches and enhance patient outcomes.
Despite these advancements, the application of AI in biofilm-targeting therapies must be accompanied by careful consideration of its limitations and ethical implications. Ensuring that these technological innovations prioritize patient safety and adhere to clinical care standards is critical for their successful integration into healthcare practices.
Zoffman et al (Zoffmann et al. 2019). employed machine learning to prioritize compounds from the Roche library based on novelty, potency, and chemical structure for antibacterial activity testing against Gram-negative bacteria. They identified compound-induced phenotypic changes and mechanisms of action, noting similarities in phenotypic fingerprints among compounds with the same mechanism of action.
Stokes et al. proposed a deep learning approach to discover antimicrobial molecules from the Drug Repurposing Hub, identifying 99 potential candidates. They empirically tested these compounds, with halicin showing strong inhibitory activity against various pathogens. Halicin's mechanism of action involves disrupting bacterial metabolism by sequestering iron and inhibiting c-Jun N-terminal kinase (Stokes et al. 2020a). Parvaiz et al (Parvaiz et al. 2021). used machine learning to search for beta-lactamase inhibitors, identifying promising compounds from a large data set. They validated 74 compounds, with one showing potential as both a β-lactam enhancer and inhibitor. Machine learning facilitated the identification of structurally similar compounds with antibacterial activity. Liu et. al (Liu et al. 2023) used deep learning to identify a novel antibiotic, abaucin, targeting Acinetobacter baumannii, a drug-resistant bacterium often found in hospitals. Conventional methods struggled to find effective antibiotics, but by screening thousands of molecules and training a neural network with the data, they discovered Abaucin.
3.4 AI Techniques and Novel Antibiotics
One prominent example is the use of graph convolutional networks to leverage the structure of molecules to translate them into graphs. Neural networks are then used to learn from the chemical structure itself, similar to how they are used to study protein structures. This approach has been successful in creating new antimicrobial compounds with desired properties (Yang et al. 2019). Recurrent neural networks [RNNs] have also been adapted to process chemical information. RNNs have also been combined with reinforcement learning to create new drug representations. RNNs have also been applied to represent protein sequences. By training RNNs on amino acid sequences, researchers can create embedded representations of proteins that can be used to predict various properties, such as protein structure and function (Alley et al. 2019).
Researchers also have used a technique called multinomial logistic regression to create a collection of useful fragments that can be combined to form new antibiotics (Mansbach et al. 2020). Another approach involves reusing existing drugs as antibiotics. In this case, scientists use a combination of neural network models to create a new way of representing chemical compounds and then assess their potential to fight microbes (Stokes et al. 2020b). There are also methods that focus on antimicrobial peptides (AMPs), which are considered a promising source of new antibiotics. Traditional machine learning models like support vector machines [SVMs] have been used to analyze AMPs and understand how they work (Lee et al. 2018). Other techniques include extreme gradient boosting, which can predict how much of a drug is needed to inhibit bacterial growth (Yan et al. 2020), and recurrent neural networks (RNNs), which can be used to design new peptide sequences with antimicrobial activity (Nguyen et al. 2019). The focus on AMPs is due to their varied mechanisms of action (MOAs), which make it harder for bacteria to develop resistance (Nagarajan et al. 2018). Researchers are using techniques like Density-based spatial clustering of applications with noise (DBSCAN)1 to group AMPs with similar properties and then testing the most promising ones in the lab (Fjell et al. 2012).
Some researchers are even combining Machine learning (ML) with traditional lab techniques. By starting with a known AMP and its close relatives, they were able to train a model to create new AMPs that are much more effective (Vishnepolsky et al. 2019). Traditionally, researchers relied on concepts like drug-likeness, encompassing factors like absorption and toxicity (ADMET), to identify promising drug candidates (Yoshida et al. 2018). ML takes this a step further by predicting binding affinity, accelerating the selection of molecules with optimal interactions with their targets (Jia et al. 2020a). Choosing the right ML algorithm for drug-likeness prediction can be challenging, as various algorithms can perform similarly. A rigorous selection process comparing multiple algorithms like Gaussian processes and neural networks is crucial to ensure optimal performance for a specific antibiotic (D'Souza et al. 2020). Beyond drug-likeness, another major hurdle in antibiotic development is potential toxicity (De Oliveira et al. 2020). ML is being harnessed to predict these risks early on, including hemolytic activity, the ability to burst red blood cells, a major concern for drugs entering the bloodstream (Jia et al. 2020b).
3.5 AI Algorithms in Prioritizing Bacterial Targets for Drug Design
AI algorithms play a transformative role in drug discovery, particularly in prioritizing bacterial targets during drug design. These algorithms apply advanced computational techniques to analyze complex datasets and rank potential drug targets based on specific criteria. This systematic approach enhances both the efficiency and accuracy of identifying viable therapeutic targets, making it a powerful tool in addressing critical challenges such as antibiotic resistance and targeted pathogen control.
3.6 Target Prioritization Approaches
- 1.
Network-Based Methods
AI-driven frameworks, like the F.O.R.W.A.R.D. (Feature-Oriented Ranking with Artificial intelligence for Rational Drug discovery) system, apply machine learning to transcriptomic data to identify molecular signatures linked to therapeutic objectives. This framework demonstrated an exceptional capability to predict target efficacy with 100% accuracy, as highlighted by Sinha et al. Such precision underscores the potential of network-based methods in revolutionizing target prioritization by integrating multiple layers of molecular data (Sinha et al. 2024).
- 2.
Genomic Target Ranking
Genomic-based approaches leverage bacterial genome analysis to prioritize targets based on their roles in essential pathways, evolutionary conservation within bacterial classes, and association with persistence mechanisms. For instance:
- ∘
Hasan et al. (2006) developed computational tools for prioritizing Mycobacterium tuberculosis targets by focusing on their metabolic significance (Hasan et al. 2006).
- ∘
Fu (2013) extended this approach, improving specificity by integrating persistence-related genes and virulence factors. These tools ensure the selection of targets that are both critical for bacterial survival and less prone to resistance development (Fu 2013).
- ∘
- 3.
In Silico Identification
Computational methods have been employed to identify bacterial proteins with minimal similarity to those in beneficial human microbiota, thereby reducing potential side effects. For example:
- ∘
Frisinger et al. (2021) demonstrated this approach by identifying essential proteins in E. coli with low homology to gut microbiota, minimizing the risk of dysbiosis. This technique exemplifies how AI can help balance therapeutic efficacy with patient safety (Svanberg Frisinger et al. 2021).
- ∘
3.7 Implications for Drug Discovery
- 1.
Accelerated Discovery
AI significantly reduces the time required to identify new antimicrobial compounds, addressing the critical need for novel antibiotics to combat rising resistance. Melo et al. (2021) highlighted how AI tools can rapidly screen vast libraries of compounds, leading to the identification of promising candidates in record time (Melo et al. 2021).
- 2.
Enhanced Specificity
AI-based prioritization enables the identification of unique bacterial targets that are essential for pathogenic survival but absent in beneficial microbiota. This selectivity can lead to the development of drugs that disrupt specific bacterial processes without harming the host microbiota.
- 3.
Advancing Personalized Medicine
With the integration of AI, personalized therapeutic regimens can be designed, focusing on patient-specific microbial profiles and genetic data, thereby improving treatment outcomes while minimizing unintended side effects.
3.8 Challenges in AI-Driven Target Prioritization
While AI offers significant advantages, challenges persist in its adoption, particularly concerning reproducibility and transparency. Computational models often operate as “black boxes,” making it difficult to interpret their predictions. Ensuring the reproducibility of results across diverse datasets and enhancing the transparency of AI algorithms are critical for their acceptance in clinical applications. Addressing these issues will be key to fully integrating AI into the drug discovery pipeline.
By utilizing AI-driven methods, researchers can not only streamline the process of drug design but also develop more precise and effective antimicrobial therapies, paving the way for breakthroughs in combating antibiotic resistance and treating infectious diseases.
For peptide-based antibiotics, solubility and stability are critical factors for success. Fortunately, ML models can predict these properties from the amino acid sequence itself, allowing researchers to focus on designing more stable and effective antibiotics (Webel et al. 2020). Furthermore, ML can identify potential cleavage sites in these peptides, which can be detrimental to their stability. By predicting these sites, researchers can optimize the peptide sequence for increased stability (Hou et al. 2020).
While ML can identify drug-like molecules, the field is now incorporating new factors. One such factor is the potential damage to the gut microbiome, a crucial consideration given the link between microbiome disruption and Antimicrobial Resistance (AMR). Researchers are developing ML models to predict how candidate antibiotics might impact the microbiome, allowing for the selection of drugs with minimal collateral damage. Ideally, these drugs would be highly specific to target pathogens while leaving beneficial gut bacteria unharmed (Torres et al. 2019).
A particularly promising area lies in exploring protein space for antibiotic development. Antimicrobial host defense peptides (AMPs) have emerged as potential antibiotic templates due to their ability to target multiple cellular sites, potentially reducing the risk of AMR. Interestingly, there seems to be an inverse relationship between resistance to traditional antibiotics and susceptibility to AMPs, suggesting further potential for crossover between ML and protein informatics in AMR research (Chng et al. 2020). Current ML models for AMR prediction primarily rely on bacterial genetic and genomic data, overlooking the drug or target molecule itself. However, research is ongoing to develop more comprehensive models that incorporate both aspects (Lázár et al. 2018).
Interpretable Machine Learning (IML) also offers exciting possibilities. By helping to identify the factors that contribute to AMR at the organismal and population level, IML can guide the development of next-generation antibiotics with lower AMR risks (Deelder et al. 2019). Analyzing AMR at the molecular and population level using ML can shed light on overused mechanisms of action [MOAs] and identify promising new avenues for antibiotic development, even on a regional scale (Kavvas et al. 2020).
Generative adversarial networks (GANs) and variational autoencoders (VAEs) are prominent architectures used in this approach. GANs involve two models: a generator creating novel molecules and a discriminator attempting to distinguish real from synthetic molecules. VAEs, on the other hand, encode and reconstruct molecules, learning the underlying data properties (Kavvas et al. 2018). Generative deep learning (DL) has broader applications in chemical and protein engineering, including material design and protein folding prediction. It's increasingly being utilized in drug discovery for generating novel drug candidates within relevant chemical spaces. Various methods employing generative DL for drug design have been explored, including deep reinforcement learning, generative adversarial autoencoders, and combinations with Monte Carlo tree search (Kingma and Welling 2014). Additionally, generative recurrent neural networks [RNNs] and Simplified Molecular Input Line Entry System (SMILES) have shown promise in drug design due to their ability to handle sequential data (Segler et al. 2018).
Jukič and Bren (Jukič and Bren 2022) surveyed the latest machine learning approaches used in the discovery of new antibacterial agents and targets, covering both small molecules and antibacterial peptides. Besides, they summarized all applied machine learning approaches and available databases useful for the design of new antibacterial agents and address the current shortcomings. Muteeb et al (Muteeb et al. 2022a). screened a library of FDA-approved drugs to identify novel non-β-lactam ring-containing inhibitors of NDM-1 by applying computational as well as in vitro experimental approaches. They employed different steps of high-throughput virtual screening, molecular docking, molecular dynamics simulation, and enzyme kinetics to identify risedronate and methotrexate as the inhibitors with the most potential. Muteeb et al (Muteeb et al. 2022b). screened a library of more than 20 million compounds, available at the MCULE purchasable database. Virtual screening led to the identification of six potential inhibitors. More recently, Lluka and Stokes (Lluka and Stokes 2023) described how advancements in AI were reinvigorating the adoption of past antibiotic discovery models—namely natural product exploration and small molecule screening.
4 Conclusion
The process of antibiotic development is slow, expensive, and prone to failure, often taking over a decade and costing millions of dollars. Between 2014 and 2019, only 14 new antibiotics were approved, and the probability of success for drugs treating infectious diseases is low, especially for orphan drugs. This risk drives corporations to prioritize research with higher returns, allowing academia to initiate early antibiotic design and optimization. Computational tools have significantly advanced drug development, enabling the rational design of bioactive compounds effective in animal models. Leveraging protein structure prediction and modeling, small-molecule antibiotic targets can be described in detail, facilitating virtual screening (VS) where compounds are docked and their binding affinity evaluated. Machine learning (ML) has greatly enhanced VS, with deep learning [DL] even bypassing docking entirely to identify antibiotics active against multiple bacterial pathogens.
Utilizing Artificial Intelligence (AI) for antibiotic design offers a promising avenue to combat the growing threat of Drug-Resistant Superbugs (DRSBs). AI can significantly accelerate the process of discovering novel antibiotics by leveraging vast datasets, predictive modeling, and innovative algorithms. Here's how AI can be leveraged for antibiotic design.
4.1 Data Mining and Analysis
AI can analyze large datasets of bacterial genomes, protein structures, and biochemical pathways to identify potential drug targets. By mining these data, AI algorithms can pinpoint vulnerabilities in pathogens that can be exploited by novel antibiotics.
4.2 Predictive Modeling
Machine learning algorithms can predict the effectiveness of potential antibiotics based on their chemical structure, pharmacological properties, and interactions with bacterial targets. This predictive modeling enables researchers to prioritize candidate compounds for further experimentation, saving time and resources.
4.3 Structure-Based Drug Design
AI algorithms can simulate the binding interactions between potential antibiotics and bacterial proteins at the atomic level. This approach, known as structure-based drug design, allows researchers to design molecules that specifically target essential bacterial proteins while minimizing off-target effects.
4.4 De Novo Drug Design
AI can generate novel antibiotic candidates from scratch using generative models and deep learning techniques. By learning from existing chemical libraries and structural databases, AI algorithms can propose entirely new molecular structures with desired antibacterial properties.
4.5 Optimization of Drug Combinations
AI can optimize combination therapy regimens by analyzing the synergistic interactions between multiple antibiotics. By considering factors such as drug pharmacokinetics, bacterial resistance mechanisms, and patient-specific variables, AI algorithms can tailor treatment regimens to maximize efficacy and minimize resistance development.
4.6 Antimicrobial Peptide Design
AI can design antimicrobial peptides (AMPs) with enhanced potency and specificity against drug-resistant bacteria. By analyzing the amino acid sequences of naturally occurring AMPs and their interactions with bacterial membranes, AI algorithms can generate novel peptide sequences with improved antibacterial activity.
4.7 Virtual Screening of Chemical Libraries
AI-driven virtual screening techniques can efficiently screen large chemical libraries to identify potential antibiotic candidates. By simulating the binding interactions between thousands of compounds and bacterial targets, AI algorithms can rapidly identify lead compounds for experimental validation.
4.8 Adaptive Learning and Evolutionary Algorithms
AI algorithms can adapt and evolve over time to keep pace with the rapidly evolving landscape of bacterial resistance. By continuously learning from new data and feedback from experimental studies, AI-driven antibiotic design platforms can iteratively improve their predictive accuracy and effectiveness.
By leveraging the capabilities of AI for antibiotic design, researchers can accelerate the discovery of novel antibiotics, overcome bacterial resistance mechanisms, and ultimately address the urgent need for effective treatments against Drug-Resistant Superbugs.
Acknowledgments
The authors have nothing to report.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Institutional Review Board Statement
The authors have nothing to report.
Endnotes
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.